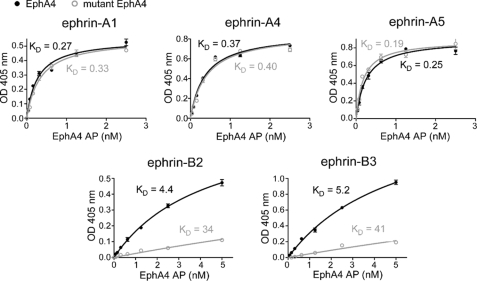
FIGURE 6.
Binding affinities of wild-type and mutant EphA4 for different ephrin ligands. Shown is the binding of different concentrations of EphA4-AP or mutant EphA4 Gln12/Glu14-AP to the indicated ephrin-Fc fusion proteins immobilized on ELISA wells. It should be noted that the Kd values for the binding of mutant EphA4 to ephrin-B2 and ephrin-B3 are approximate because mutant EphA4-AP concentrations sufficiently high to achieve maximal binding to these ephrins could not be achieved due to the low binding affinity. The curves for mutant EphA4-AP were fitted to the maximal binding value (Bmax) calculated from the wild-type EphA4 binding curves (Bmax = 0.55 for ephrin-A1, 0.86 for ephrin-A4, 0.90 for ephrin-A5, 0.88 for ephrin-B2, and 1.9 for ephrin-B3). In the case of the A-type ephrins, the same Bmax values were obtained by fitting the curves for mutant EphA4 without constraining Bmax.