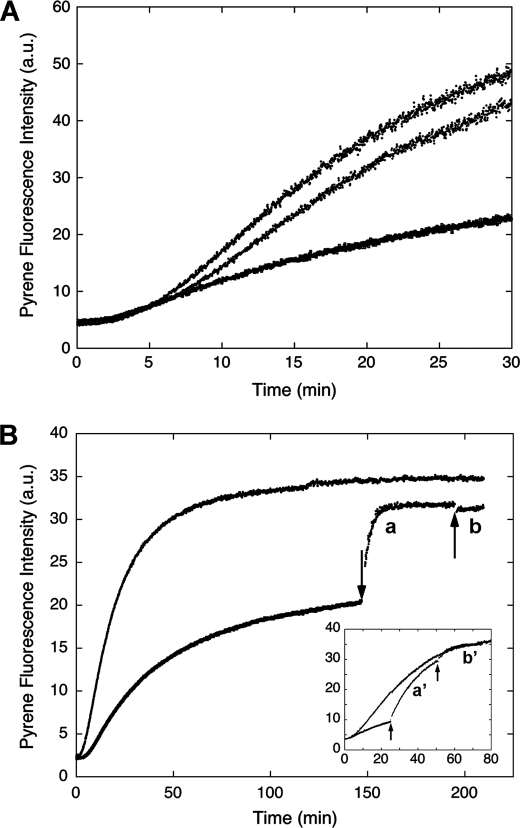
FIGURE 3.
Effect of CaM on the pyrene-actin fluorescence change in the presence of H32K. A, when added at the beginning of actin polymerization, inclusion of Ca2+/CaM (middle trace) neutralizes the effect of H32K (bottom trace) on the pyrene-actin fluorescence. Actin alone is represented by the top trace. Concentrations: actin, 1.5 μm; H32K, 0.5 μm; CaM, 3 μm. B, reversible binding of H32K during actin polymerization in ATP-containing F-buffer. H32K (0.5 μm) was added at the beginning of actin (1.5 μm) polymerization as described (bottom trace). At t = 150 min (indicated by arrow) when polymerization approached steady state, CaM (3 μm) was added to displace the actin-bound H32K (trace a); the fluorescence rise fit first-order kinetics with a rate constant of 0.288 ± 0.004 min−1. At t = 195 min EGTA (2 mm) was added to chelate Ca2+ (trace b). The top trace is for actin alone. Inset, earlier addition of CaM (at t = 25 min) resulted in a slower transition (the apparent first-order rate constant was 0.0544 ± 0.0004 min−1), as actin continued to polymerize; when EGTA was added (at t = 50 min), H32K was able to re-bind the post-transitional actin filament causing further acceleration of polymerization. The same protein concentrations and trace labeling (i.e. a′ corresponds to a; b′ corresponds to b) apply to the inset. All fluorescence readings were corrected for dilution effects.