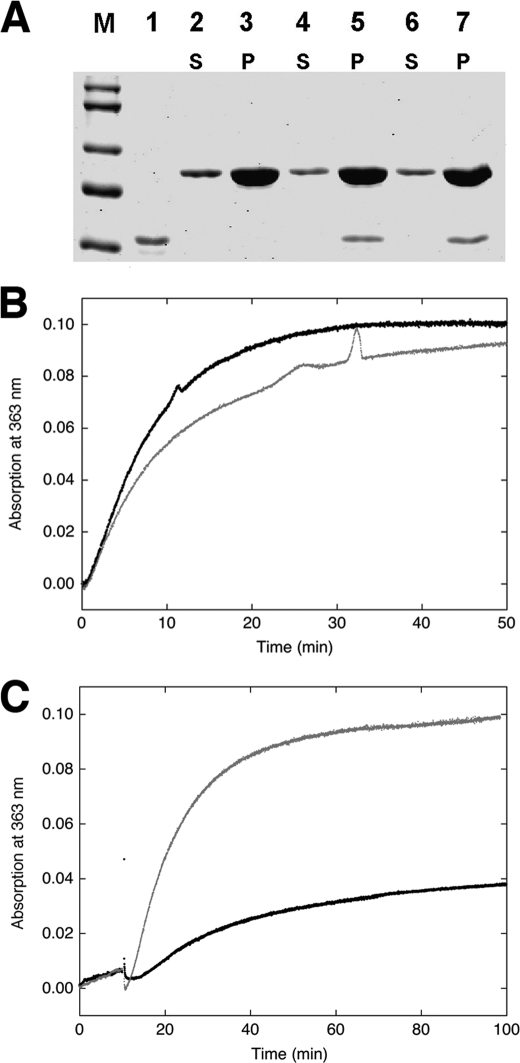
FIGURE 4.
Effect of CaD on actin polymerization. A, SDS-PAGE of H32K co-sedimented with actin polymerized for 45 min without (lanes 2 and 3) or with H32K added either at the beginning (lanes 4 and 5) or 18 min after the initiation (lanes 6 and 7) of polymerization. M, molecular markers; lane 1, H32K alone. S and P are supernatant (1/20 of total sample loaded) and pellet (1/2 of total sample loaded) fractions, respectively. Protein concentrations are the same as in Fig. 1. B, polymerization monitored by phosphate release of actin alone (black trace) and actin with H32K added at the beginning of reaction (gray trace). Generation of Pi was monitored continuously at room temperature (see “Experimental Procedures”). The baseline, which was obtained for each set of experiments under identical conditions except in the absence of actin, was subtracted from the experimental reading. Protein concentrations used: 12 μm actin and 2 μm H32K. C, comparison between polymerization of actin alone (12 μm) in the presence of F-buffer containing ATP (gray trace) or ADP (black trace), monitored by phosphate release. The residual activity for the ADP sample may have resulted from contamination of ATP.