Abstract
Modulation of K+ conductance of the inner mitochondrial membrane has been proposed to mediate preconditioning in ischemia-reperfusion injury. The mechanism is not entirely understood, but it has been linked to a decreased activation of mitochondrial permeability transition (mPT). In the present study K+ channel activity was mimicked by picomolar concentrations of valinomycin. Isolated brain mitochondria were exposed to continuous infusions of calcium. Monitoring of extramitochondrial Ca2+ and mitochondrial respiration provided a quantitative assay for mPT sensitivity by determining calcium retention capacity (CRC). Valinomycin and cyclophilin D inhibition separately and additively increased CRC. Comparable degrees of respiratory uncoupling induced by increased K+ or H+ conductance had opposite effects on mPT sensitivity. Protonophores dose-dependently decreased CRC, demonstrating that so-called mild uncoupling was not beneficial per se. The putative mitoKATP channel opener diazoxide did not mimic the effect of valinomycin. An alkaline matrix pH was required for mitochondria to retain calcium, but increased K+ conductance did not result in augmented ΔpH. The beneficial effect of valinomycin on CRC was not mediated by H2O2-induced protein kinase Cϵ activation. Rather, increased K+ conductance reduced H2O2 generation during calcium infusion. Lowering the osmolarity of the buffer induced an increase in mitochondrial volume and improved CRC similar to valinomycin without inducing uncoupling or otherwise affecting respiration. We propose that increased potassium conductance in brain mitochondria may cause a direct physiological effect on matrix volume inducing resistance to pathological calcium challenges.
Introduction
A short non-injurious ischemic insult can greatly reduce the severity of a subsequent prolonged ischemia, a phenomenon generally referred to as preconditioning or ischemic tolerance (1–3). This adaptive response can be seen as a general biological phenomenon by which organisms respond with protective mechanisms to potentially recurring challenges (4). A low dose of one type of stressful stimulus can also induce resistance to another, e.g. prior transient hyperthermia can protect against subsequent forebrain ischemia (5). Understanding and controlling these seemingly general endogenous survival responses might enable a clinical therapeutic opportunity to reduce tissue damage after cerebral or cardiac ischemia (4, 6, 7). Ischemic preconditioning mediates both a rapid adaptive response coming into effect within minutes to hours as well as an induced tolerance occurring over a longer time frame requiring gene activation and de novo protein synthesis. The former is extensively studied in cardiac ischemia and the latter in cerebral ischemia (6, 7). Various physiological and chemical triggers can induce preconditioning, and whereas several mediating pathways have been characterized, the final causal effectors remain more obscure (7).
The inner mitochondrial membrane has been proposed to contain ATP-sensitive potassium channels (mitoKATP),2 which are pharmacologically distinct from plasma membrane KATP and may mediate as well as be end-effectors of the preconditioning effect. Although there is substantial support for mitoKATP activation in preconditioning, the evidence comes almost exclusively from studies with pharmacological compounds, mainly diazoxide and 5-hydroxydecanoate (7, 8). Ischemic preconditioning can be closely mimicked by the KATP opener diazoxide, which shows a concentration-dependent selectivity for mitoKATP over plasma membrane KATP (9, 10). Both ischemic and diazoxide-mediated preconditioning can be blocked by 5-hydroxydecanoate, which is believed to inhibit the diazoxide-induced opening of mitoKATP (11). A role of mitoKATP is also implicated in different models of cerebral ischemia where pretreatment with diazoxide mediates neuronal protection in a 5-hydroxydecanoate-sensitive manner (12, 13).
Activation of the mitochondrial permeability transition (mPT) phenomenon is considered to participate in cell death pathways associated with various types of pathological stimuli, particularly ischemia-reperfusion injury (14–16). The mPT is also implicated in the pathogenesis of several acute or chronic neurodegenerative diseases, substantiated by the neuroprotective effects of cyclosporin A, its non-immunosuppressive analogs, or genetic deletion of the mPT component and cyclosporin A target cyclophilin D in animal models (17–19).
Ischemic preconditioning and mitoKATP channel activation have been proposed to afford tissue protection by inhibiting mPT activation during reperfusion (8, 10, 20, 21). A signaling pathway linking mitoKATP and mPT has been proposed where the increased K+ conductance after mitoKATP opening alkalinizes the mitochondrial matrix and increases generation of H2O2, which in turn activates an mPT-associated PKCϵ (22, 23). An alkaline matrix pH has also been put forward as the basis for efficient mitochondrial calcium buffering (24). Furthermore, prevention of matrix contraction by K+-mediated volume effects may preserve mitochondrial respiratory capacity and energy transfer during ischemia-reperfusion (25–27).
The aims of the present study were to (i) investigate if increased K+ conductance modulates calcium retention capacity (CRC) and activation of mPT in brain mitochondria and (ii) to explore the physiological effects of increased K+ conductance and determine the mechanism by which activation of mitochondrial K+ channels improves mitochondrial resistance to calcium challenges in neuronal preconditioning.
EXPERIMENTAL PROCEDURES
Isolation of Brain Mitochondria
Adult male Wistar rats (Harlan Scandinavia ApS, Allerød, Denmark) of 325–400 g were allowed ad libitum access to water and food before use. Animals were normally decapitated after a brief exposure to halothane to minimize stress, but one animal for each experimental setting was decapitated without gaseous anesthesia to ensure that the process did not influence the results. All animal procedures were approved by the Malmö/Lund Ethical Committee for Animal Research (M221-03, M44-07). Isolation of cortical non-synaptosomal brain mitochondria was achieved by using a discontinuous Percoll gradient as described previously (28, 29). All steps were carried out under ice-cold conditions. Protein quantification of mitochondrial suspensions was performed by the Bradford method using bovine serum albumin as standard.
Mitochondrial CRC, NAD(P)H Fluorescence, Membrane Potential (ΔΨm), Light Scattering, and H2O2 Generation
A luminescence spectrometer LS-50B (PerkinElmer Life Sciences) with a temperature controlled cuvette holder (37 °C) was used for all fluorescence and light-scattering experiments. Mitochondria (100 μg/2 ml) were suspended in 125 mm KCl, 20 mm Tris buffer, pH 7.1, containing 2 mm Pi (K+), 1 mm MgCl2, 1 μm EGTA, 200 μm ATP, and 5 mm NADH-linked respiratory substrates malate and glutamate. After the addition of mitochondria, 1 μg/ml oligomycin, 50 μm ADP, and experimental compounds were added. In experiments evaluating matrix volume effects on CRC, the osmolarity of the buffer was decreased by lowering the KCl concentration from 125 to 75 mm. Mitochondrial calcium uptake and release were monitored by the excitation ratio (excitation 340/380 nm, emission 509 nm) of the extramitochondrial calcium-sensitive fluorescent probe Fura 6F (250 nm). The mitochondrial suspensions were infused with 200 nmol of CaCl2/(mg × min)(10 μm/min). The start of calcium uptake was defined as the point where the experimental curve deviated from a control curve with 1 μm ruthenium red, blocking mitochondrial calcium uptake through the uniporter. CRC was calculated as the amount of infused calcium from the start of mitochondrial calcium uptake until the start of maximal calcium release. The redox status of NAD(P)H was determined qualitatively by following autofluorescence of NAD(P)H with excitation at 340 nm and emission at 460 nm during calcium infusions. ΔΨm was assessed qualitatively by following fluorescence quenching of rhodamine 123 (100 nm), excitation and emission at 490 and 528 nm. Light scattering of mitochondria was detected at 90° and measured at 520 nm. Mitochondrial H2O2 generation was detected by following the oxidation of 1 μm Amplex Red to the fluorescent product resorufin in the presence of horseradish peroxidase and superoxide dismutase (0.5 and 20 units/ml, respectively). Excitation was set to 560 nm and emission to 590 nm. Known amounts of H2O2 were added to establish a calibration curve.
High Resolution Respirometry
Oxygen consumption of mitochondria during calcium infusion was measured under similar conditions as Fura 6F experiments described above except for the continuous decline in oxygen concentration in the sealed chamber. Also, 10 μm bovine serum albumin was present in the buffers of the experiments comparing respiration and CRC in 75 and 125 mm KCl. Studies were performed in an Oroboros Oxygraph-2k with a Titration-Injection microPump TIP-2k using DatLab 4 software allowing online respiration rate output with high sensitivity, low noise, and concentration-dependent background correction (Oroboros Instruments, Innsbruck, Austria) (30). CRC in the respiration experiments was calculated as the amount of infused calcium from the start of elevated respiration rate until the start of rapid respiration decrease.
Matrix pH Measurements
Changes in intramitochondrial pH were measured by determining the equilibrium distribution of the weak acid acetate (31). The uncharged protonated species (HAc) equilibrates over the mitochondrial membranes. The anion (Ac−) is impermeant and, therefore, accumulates in an alkaline compartment, such as the mitochondrial matrix, due to continued dissociation and uptake of HAc. The pH difference can be determined by the relationship ΔpH = log10 Acext−/Acm−, where ext and m refers to extra-matrix and matrix compartments, respectively. A whole preparation of brain mitochondria (∼700 μg) was divided in half and incubated in the ordinary KCl media supplemented with 3H-labeled acetate (0.5 μCi or 18.5 kBq/ml) and 14C-labeled sucrose (0.05 μCi or 1.85 kBq/ml) and 1 mm “cold” acetate and sucrose. [14C]Sucrose does not permeate the inner mitochondrial membrane and was used to correct for non-matrix 3H activity. After incubation with either 3 pmol/mg valinomycin or vehicle (ethanol) in the presence of adenonucleotides and respiratory substrates (see above), the suspension was carefully layered on top of 900 μl of silicone oil AR 110 and centrifuged at 20,800 × g for 2 min, leaving the mitochondria in a pellet and the aqueous phase above the silicone oil. Half of the supernatant (500 μl) was transferred to new vials. The remaining supernatant and silicone oil was carefully but rapidly removed, and the mitochondrial pellet was lysed in 500 μl of H2O. One series of experiments was also performed without filtering through silicone oil. Both pellet and supernatant samples were deproteinized with perchloric acid (2% w/v final) (32), and the precipitate was spun down. Two samples (2 μl) of supernatant and 500 μl of the clear pellet solution were transferred to scintillation vials (HDPE 24 ml, VWR) with 5 ml of Ready Safe scintillation fluid (Beckman Coulter, Fullerton, CA) and H2O (10:1 final). Samples were measured in a LS 6500 Scintillation counter (Beckman Coulter). Similar experiments were performed with [3H]H2O for matrix volume estimates but did not yield reliable values as the signal to noise ratio was far inferior to that of tritiated acetate. For ΔpH calculations, a matrix volume of 1 μl/mg was assumed (33).
Statistical Analyses
All experiments were replicated in at least four separate mitochondrial preparations. Average results are presented as the mean ± S.D. and were evaluated with one-way ANOVA followed by the Bonferroni post hoc test. The level of statistical significance was set to 5%.
RESULTS
Increased K+ Conductance Induced by Low Concentrations of Valinomycin Increases the CRC of Brain Mitochondria
The lipophilic K+ carrier valinomycin induces an increased K+ conductance of the inner mitochondrial membrane and translocates K+ from the intermembrane side to the matrix side electrophoretically as a result of the mitochondrial membrane potential (ΔΨm, inside negative) (34). In the present study, valinomycin was administered at low concentrations to mimic K+ channel activity, and the effect on physiological parameters of brain mitochondria such as oxygen consumption, ΔΨm, light scattering, NAD(P)H fluorescence, and the ability to sequester calcium was assayed. Valinomycin at 150 pm (3 pmol/mg of mitochondria) significantly increased the CRC of brain mitochondria exposed to a continuous infusion of calcium (Figs. 1 and 3). Parallel to the CRC assay, during calcium infusion, valinomycin prolonged the delay before a rapid decrease of NAD(P)H fluorescence and oxygen consumption (data not shown and Fig. 2, respectively). The dose-response relationship demonstrated a significant increase of CRC at 150–300 pm, whereas higher concentrations gradually attenuated the positive effect, and 2 nm valinomycin decreased CRC (Fig. 3).
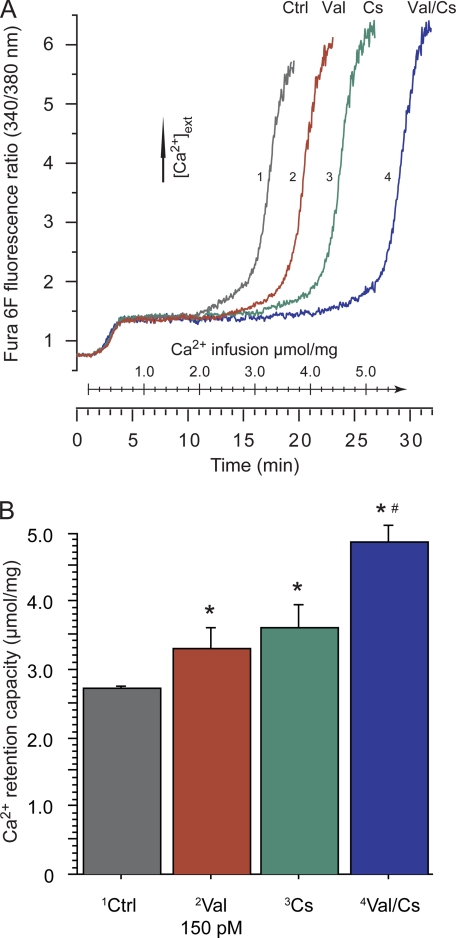
FIGURE 1.
Increased K+ conductance by valinomycin and inhibition of cyclophilin D separately and additively increase brain mitochondrial calcium retention capacity. Suspensions of isolated brain mitochondria in KCl-based buffer (100 μg/2 ml) were infused with 200 nmol of Ca2+/(mg × min) (10 μm Ca2+/min). Experiments were run in the presence of 200 μm ATP, 1 μg/ml oligomycin, 50 μm ADP, and 5 mm malate and glutamate. Additions were made with 150 pm K+ carrier valinomycin (Val) and/or 1 μm non-immunosuppressive cyclosporin analog D-MeAla3EtVal4-cyclosporin (Cs, also called Debio-025 or UNIL025). Vehicle (0.2% ethanol) was present in control runs (Ctrl). A, CRC of brain mitochondria was determined by following the fluorescence ratio of Fura 6F (250 nm) reflecting extramitochondrial Ca2+ concentration. A plateau level is reached when mitochondrial calcium uptake equals the rate of infused calcium, and rapid calcium release occurs at a certain threshold considered to reflect activation of mPT. B, CRC was calculated as the amount of infused calcium from the start of calcium uptake (the time point where the experimental curve deviated from a control curve with inhibited calcium uptake) to the start of maximal calcium release. Values are the means ± S.D. (μmol Ca2+/mg mitochondria) of four separate mitochondrial preparations. The asterisk (*) indicates significant difference compared with control (vehicle run), and the number symbol (#) indicates significant combined effect of the valinomycin and D-MeAla3EtVal4-cyclosporin compared with each of the drugs separately.
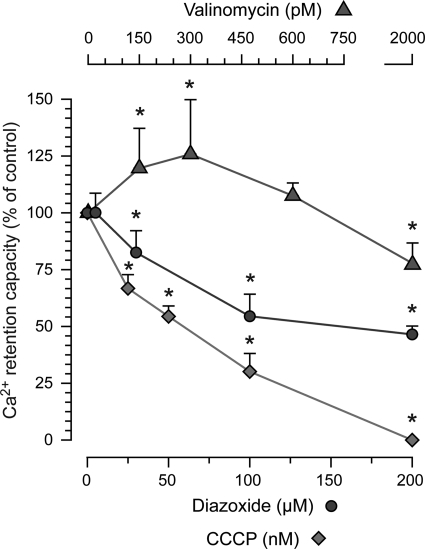
FIGURE 3.
Dose-related effects of increased K+ conductance and H+ conductance or diazoxide on brain mitochondrial calcium retention. Experiments were run as described in Fig. 1, and CRC is expressed as % of control (vehicle, 0.2% ethanol or DMSO). The K+ carrier valinomycin increased CRC at low concentrations, but a decrease was seen at the highest tested concentration. In contrast, all tested concentrations of the protonophore CCCP dose-dependently decreased CRC. The K-ATP channel opener diazoxide was without effect at 5 μm, but higher concentrations decreased CRC. The asterisk indicates significant difference compared with control.
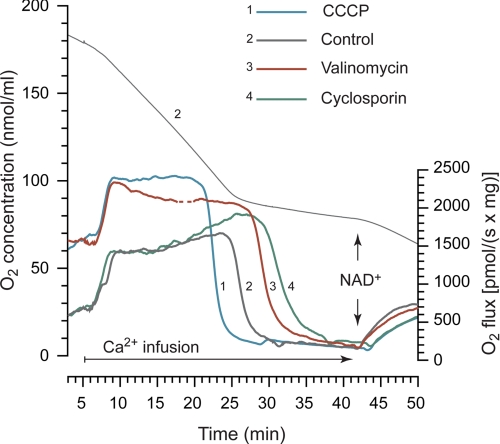
FIGURE 2.
High resolution respirometry of brain mitochondria during calcium uptake and release. Experiments were performed as described in Fig. 1, except mitochondrial suspensions were analyzed in air-tight chambers. Oxygen consumption of mitochondria was determined during calcium infusion, and the upper trace displays the O2 concentration (nmol/ml) in the chamber for a representative control experiment. The lower traces show the real-time O2 consumption rate (pmol O2/s × mg mitochondria) of vehicle (Control), 150 pm valinomycin, 1 μm D-MeAla3EtVal4-cyclosporin, or 25 nm protonophore CCCP. Basal respiration rates were significantly higher in the presence of valinomycin or CCCP. In all runs calcium infusion induced an increased rate of respiration from basal levels followed by a rapid decrease in respiration, attributed to permeability transition. To confirm altered inner membrane permeability, 150 μm NAD+ was administered, which increased respiration in all groups after calcium infusion. Traces are representative examples of four separate experiments. Disturbances because of air inlet in the chamber have been deleted in trace 3 (dashed segment). Quantification of CRC is provided in Figs. 1 and 3.
Increased H+ Conductance by the Protonophore CCCP or Electroneutral K+/H+ Exchange by Nigericin Reduces the Calcium Retention Capacity of Brain Mitochondria
In contrast to the effect of valinomycin, increased H+ conductance of the inner mitochondrial membrane by the protonophore CCCP dose-dependently decreased CRC over all tested concentrations and completely abolished CRC at 200 nm (Fig. 3). The lowest tested concentrations of CCCP (25 nm) and valinomycin (150 pm) both significantly increased basal respiration rate from control (1454.1 ± 202, 1449.5 ± 257, and 647.6 ± 150 pmol O2/(s × mg), respectively), and there was no significant difference between the compounds (Fig. 2). Thus, even though the compounds displayed similar degrees of uncoupling, they exhibited opposite effects on CRC. The electroneutral K+/H+ exchanger nigericin (5 nm) hyperpolarized the mitochondria (data not shown) and significantly decreased the CRC of brain mitochondria suspended in a buffer with physiological pH (Fig. 5A). An increase in ΔΨm was, therefore, not sufficient to increase CRC per se.
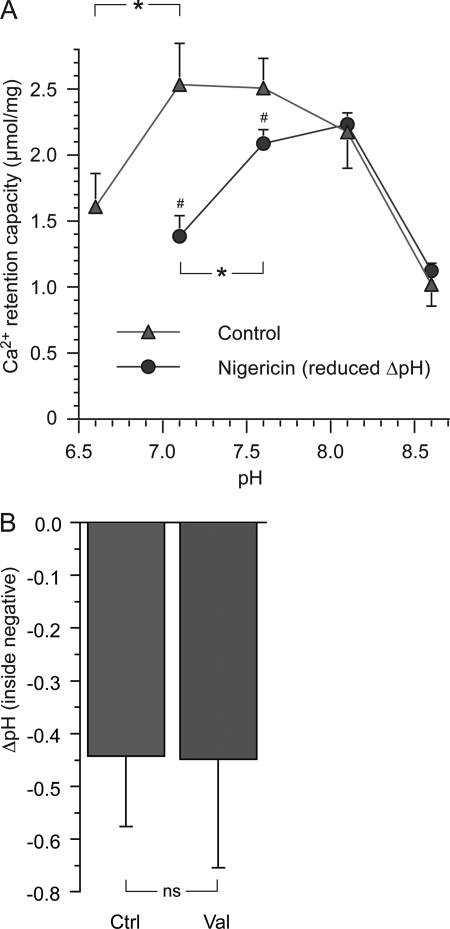
FIGURE 5.
The influence of pH on mitochondrial calcium retention and measures of ΔpH. A, experiments were performed as in Fig. 1. The pH of the buffer was modified between 6.6 to 8.6, and for indicated groups, the pH gradient over the inner mitochondrial membrane (ΔpH, normally matrix alkaline) was reduced by the electroneutral K+/H+ exchanger nigericin (5 nm). A neutral or slightly alkaline pH was optimal for CRC in control mitochondria, whereas pH 7.6–8.1 was most beneficial for mitochondria with reduced ΔpH. The asterisk (*) indicates significant difference between groups, and the number symbol (#) indicates significant difference of nigericin compared with control. B, shown are calculations of ΔpH using equilibrium distribution of tritiated acetate. Calculations were based on quench-corrected and 14C-subtracted 3H dpm in mitochondrial supernatants or pellets from mitochondria incubated with [3H]acetate and [14C]sucrose with or without 3 pmol/mg valinomycin (Val). ns indicates no significant difference in ΔpH between groups (n = 12).
Permeability Transition Limits the Extent of Mitochondrial Calcium Retention
mPT is classically assayed in light-scattering assays measuring matrix volume or configuration changes. Activation of mPT is usually detected after large bolus additions of calcium or by calcium loading followed by a second inducing agent such as an oxidant or protonophore. Although the mitochondria behave as though they have certain individual probabilities to undergo mPT, after a large bolus of calcium the mPT becomes more of an all-or-nothing phenomenon for the whole mitochondrial population. A compound that slightly influences the threshold for mPT activation or interferes with the calcium loading can, therefore, display a dramatic effect. Recently, more quantitative assays have been introduced where either small repetitive calcium doses or, as in the present study, a continuous infusion of calcium is administered. The strengths of these latter assays are a lesser degree of sudden bioenergetic demand for the mitochondria (24) and a quantitative evaluation of both positive and negative effects of a tested treatment. The measurement of extramitochondrial calcium provides a functional assay for mitochondrial calcium uptake (Fig. 1A). The rapid calcium release after a prolonged period of calcium uptake occurs concomitantly with a decrease in light-scattering and NAD(P)H fluorescence in brain mitochondria and has, therefore, been attributed to activation of mPT (24). However, the calcium-phosphate complexes formed within the matrix during calcium loading interfere with light-scattering and NAD(P)H fluorescence, and changes in these parameters are not necessarily associated with mPT (35). To obtain a more specific correlation between CRC and mPT, the calcium infusion experiments were repeated in airtight chambers during measurements of oxygen consumption (Fig. 2). Mitochondrial respiration increased during active calcium uptake and retention, but at the time, corresponding to rapid calcium release, respiration was severely decreased. The respiratory inhibition was attributed to loss of NAD(H) from the matrix after mPT. The addition of exogenous NAD+, which is impermeant to the inner mitochondrial membrane in intact mitochondria, increased respiration after calcium infusion, indicating that the mitochondria had undergone mPT (Fig. 2). It was not necessary to supplement cytochrome c in these experiments to reveal the stimulation of respiration by NAD+ after mPT, but it may be necessary in mitochondria isolated from other organs (36). Further identifying the mPT as the limiting factor for mitochondrial CRC is the finding that the cyclophilin D inhibitor D-MeAla3EtVal4-cyclosporin (Debio-025) increased CRC independently and additively to valinomycin (Figs. 1 and 2).
Calcium Retention Capacity of Brain Mitochondria Is Dependent on an Alkaline Matrix pH
A low pH is considered to stabilize the mPT pore complex in a closed configuration. This is apparent in mPT assays run under de-energized conditions (37, 38), i.e. under conditions where active calcium uptake and retention is inhibited. The beneficial effect of acidic pH in inducing resistance toward mPT was confirmed for de-energized brain mitochondria in the present study (data not shown) and has also been demonstrated by others (39). In contrast, under physiological conditions, an alkaline mitochondrial matrix is considered to be the basis for efficient calcium complexation with phosphate forming inactive calcium phosphates and, thus, keeping free [Ca2+] at a regulated low level (40) (Fig. 4). To further study the role of pH on mitochondrial calcium accumulation and resistance to mPT, calcium infusion experiments were run over a wide pH range (Fig. 5A). An acidic pH (pH 6.6) was detrimental for CRC, as were extreme alkaline conditions (pH 8.6). Neutral or slightly alkaline conditions were most favorable for the retention of calcium in brain mitochondria (pH 7.1–7.6). In contrast, mitochondria with a reduced ΔpH (treated with the electroneutral K+/H+ exchanger nigericin) displayed the largest CRC at pH 7.6–8.1. Thus, with a nigericin-induced reduction in ΔpH, a more alkaline pH is required for efficient calcium retention.
FIGURE 4.
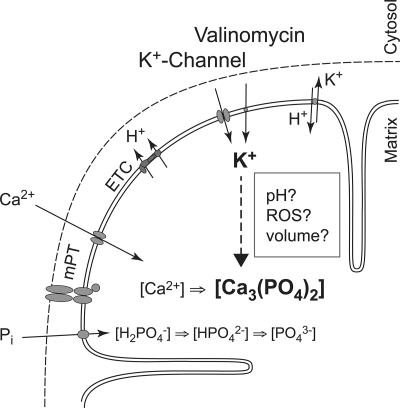
How increased K+ conductance may improve mitochondrial calcium retention and sensitivity to permeability transition. Schematic illustrations are shown of some proposed physiological effects caused by increased K+ conductance under conditions where mitochondria buffer an increased cytoplasmic calcium load. Increased K+ conductance by K+ channel activation or by the K+-carrier valinomycin may enhance matrix alkalization via increased H+ extrusion by the electron transport chain (ETC). The uptake of phosphate (Pi) and the equilibrium between differently protonated phosphate groups each depends on pH. An alkaline pH enhances the availability of PO43−, enabling formation of calcium phosphate complexes (e.g. Ca3(PO4)2) from free Ca2+. Enhanced matrix volume due to the osmotic effect of K+ may also lead to increased availability of Pi and enhanced calcium complex formation. The maintenance of low [Ca2+] in the mitochondrial matrix reduces the sensitivity of mPT and may, therefore, increase CRC. Increased K+ conductance has further been suggested to elevate mitochondrial ROS production, which activates PKCϵ and, through its claimed association with the mPT pore complex, induces resistance to mPT (22, 44, 53).
Increased K+ Conductance Induced by Valinomycin Does Not Cause Increased Alkalization or Reduced Free [Ca2+] in the Mitochondrial Matrix
One possible mechanism whereby increased K+ conductance can enhance CRC is elevation of matrix pH (Fig. 4). A more alkaline matrix lowers free [Ca2+] through increased uptake and deprotonation of phosphate with a resulting increase in calcium-phosphate complexation. To test this hypothesis, intramitochondrial pH was measured in a repeated series of experiments using equilibrium distribution of tritiated acetate. The radioactivity from tritiated acetate used to calculate ΔpH was not significantly different in control and valinomycin-treated mitochondria. Experiments where mitochondria were filtered through silicone oil yielded somewhat higher values of ΔpH than experiments without filtration (−0.54 ± 0.12 and −0.39 ± 0.11 for the respective control runs). A summary of the results with all experiments included is presented in Fig. 5B.
Furthermore, there was no indication that increased K+ conductance caused reduced matrix [Ca2+]. Extramitochondrial [Ca2+] was not affected during calcium loading (Fig. 1). In calcium set-point experiments (40), the calcium infusion was halted, and extramitochondrial [Ca2+] reached a new somewhat lower equilibrium level (data not shown). After the addition of small amounts of the calcium chelator EGTA in the presence of 18 mm NaCl, mitochondria gradually released Ca2+ and regained a similar equilibrium level of extramitochondrial [Ca2+] as before calcium chelation, i.e. the mitochondrial calcium set-point concentration (data not shown). As the activity of mitochondrial Na+/Ca2+ exchanger is dependent on ΔpH and matrix [Ca2+] (41, 42), a difference in these parameters between control and valinomycin-treated mitochondria would expectedly influence the calcium set-point concentration as well as the rate of efflux, but no such differences were observed.
Increased Calcium Retention by Valinomycin Is Not Dependent on Increased ROS and the Associated Activation of PKCϵ
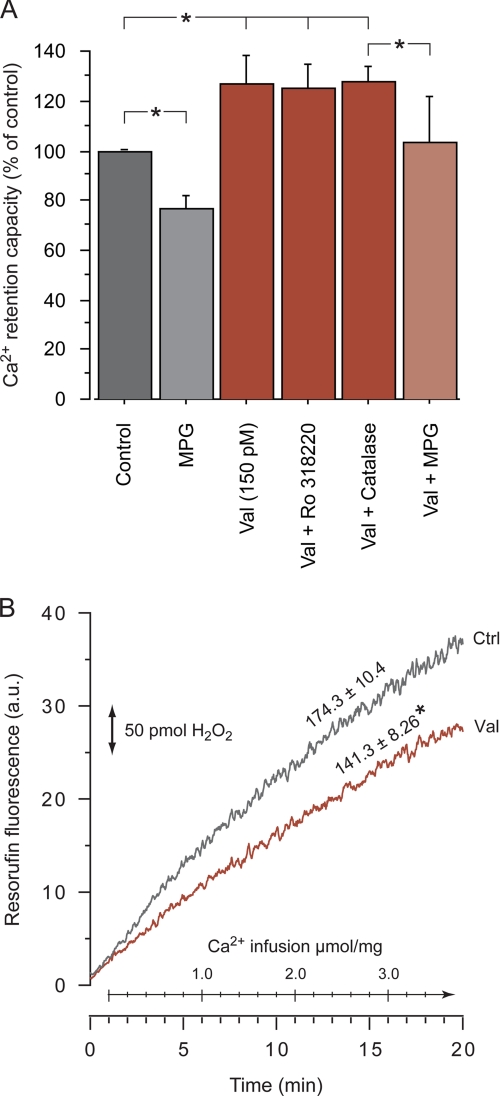
Garlid and co-workers (23) have described a pathway in isolated heart mitochondria by which increased K+ conductance through the opening of mitoKATP or by valinomycin triggers an increase in H2O2. Hydrogen peroxide in turn activates PKCϵ, which associates with and inhibits mPT. They showed that openers of mitoKATP or valinomycin inhibited calcium-induced mPT, but the protection could be blocked by the PKC inhibitor Ro318220 and the ROS scavenger N-2-mercaptopropionylglycine (MPG) (23). In the present study, Ro318330 (0.1 μm) did not influence the valinomycin-mediated increase of brain mitochondrial CRC (Fig. 6A). MPG (300 μm) decreased CRC both in control and valinomycin-treated mitochondria, whereas the antioxidant catalase (2000 units/ml) had no effect, indicating an unspecific disadvantageous effect of MPG unrelated to ROS scavenging. To further study a possible contribution of ROS generation to the beneficial effect of valinomycin on CRC, H2O2 generation was monitored during the calcium infusion experiments (Fig. 6B). The oxidation rate of Amplex Red to the fluorescent product resorufin was significantly decreased in the presence of 150 pm valinomycin. Furthermore, successive additions of 50 pm (1 pmol/mg of mitochondria) valinomycin to brain mitochondria without infused calcium lowered basal H2O2 generation in a dose-dependent manner (data not shown). Hence, increased ROS production and activation of PKCϵ did not mediate the increased capacity of brain mitochondria to accumulate calcium in the present study.
FIGURE 6.
Increased calcium retention by valinomycin is not blocked by PKC inhibition or associated with increased H2O2 generation. A, shown is CRC of brain mitochondria during a continuous calcium infusion as described in Fig. 1. Mitochondria with 150 pm valinomycin were pretreated with the PKC inhibitor Ro318220 (100 nm), the free radical scavenger MPG (300 μm), or 2000 units/ml of the antioxidant catalase. MPG reduced CRC with and without valinomycin, indicating a nonspecific negative effect. The asterisks indicate significant difference between groups. B, H2O2-dependent oxidation of Amplex Red to the fluorescent product resorufin was followed during calcium infusion for control (Ctrl) and valinomycin-treated (Val, 150 pm) brain mitochondria. Inserted numbers display mean H2O2 generation rate in pmol/min/mg. The asterisk indicates significant difference compared with control. a.u., arbitrary units.
Increasing Matrix Volume Enhances Calcium Retention Capacity without Affecting Respiratory Activity
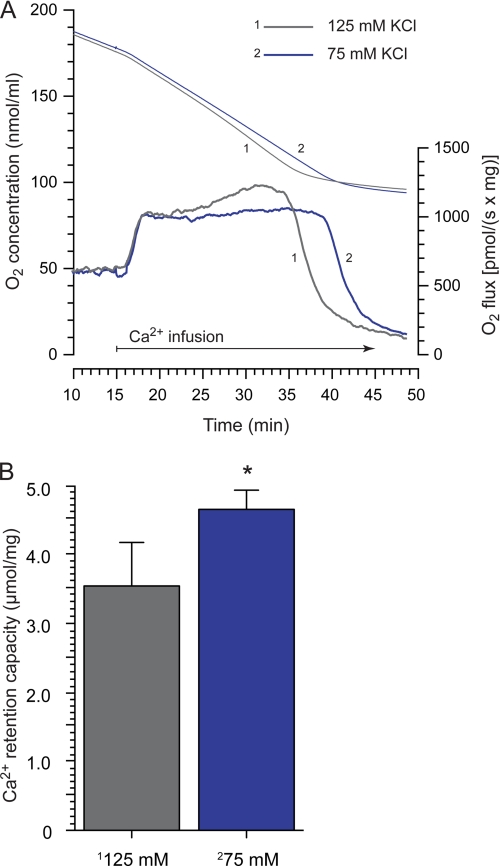
To investigate effects on matrix volume by increased K+ conductance and to separate the volume effects from depolarization and uncoupling in relation to CRC, the osmolarity of the suspending buffer was reduced (by reducing the KCl concentration from 125 to 75 mm). Volume effects were estimated by assaying 90° light scattering, and the nonspecific ionophore alamethicin was used to obtain a standardized maximal swelling response (data not shown). The reduced KCl concentration and 150 pm valinomycin, both, reduced light scattering significantly (12.76 ± 2.2 and 17.1 ± 7.1%, respectively, n = 5–8) with no significant difference between the treatments. Calculations of CRC demonstrated a significant increase in CRC in mitochondria suspended in 75 mm compared with 125 mm KCl (Fig. 7). The reduced KCl concentration did not, however, significantly affect respiratory rates before or during calcium uptake (n = 5, representative traces demonstrated in Fig. 7A).
FIGURE 7.
The influence of matrix volume on brain mitochondrial calcium retention. Conditions were as described in Figs. 1 and 2 except that 10 μm bovine serum albumin was present. A, respirometry of mitochondria suspended in standard (125 mm, trace 1) or reduced (75 mm, trace 2) concentrations of KCl during a continuous calcium infusion are shown. The KCl concentration was reduced to lower the medium osmolarity and thereby increase matrix volume. Upper traces are representative examples of changes in O2 concentration, and lower traces depict the corresponding respiration rates (n = 5). The rapid decrease in respiration rate indicates activation of permeability transition. There were no significant differences in basal or calcium-stimulated respiration rates, but there was significant difference in time to respiratory inhibition. B, quantification of CRC of brain mitochondria suspended in 125 or 75 mm KCl media. CRC was calculated as the amount of infused calcium from the start of the elevated respiration rate until start of rapid respiration decrease. The asterisk indicates significant difference between groups.
The increase in respiration induced by valinomycin was attributed to K+ cycling and not to volume differences (neither nigericin nor the changes in osmolarity above influenced respiration). The augmented oxygen consumption during mitochondrial calcium accumulation was largest at the start of infusion with a gradual decline corresponding to a K+ flux of 900 to 350 nmol of K+/(min × mg). Similar flux rates were obtained in the absence of active calcium uptake.
The MitoKATP Channel Opener Diazoxide Does Not Mimic the Effect of Valinomycin and Reduces Calcium Retention
The putative mitoKATP channel opener diazoxide was tested at 5–200 μm for effects on CRC. Care was taken to follow previously reported conditions for increasing probability of mitoKATP detection, i.e. ATP was present in solution before mitochondria and diazoxide dissolved in DMSO was added immediately after mitochondria to ensure even distribution (22). However, one discrepancy was the time frame of the present experiments compared with typical assays detecting mitoKATP, which usually monitor the first few minutes after the addition of mitochondria from isolation medium to experimental buffer (22). Diazoxide did not induce any light-scattering changes, in contrast to valinomycin, which induced readily detectable decreases already at 50 pm (data not shown). Whereas 5 μm diazoxide had no effect on mitochondrial calcium handling, 30–200 μm dose-dependently decreased CRC (Fig. 3). The negative effects of diazoxide were probably related to unspecific effects on mitochondria such as uncoupling of mitochondria at higher doses (43).
Heart Mitochondria
To evaluate the generability of the findings in brain mitochondria, key experiments were performed in isolated heart mitochondria. A similar correlation between matrix volume and CRC was found where an increased matrix volume increased CRC. Aggravated respiratory uncoupling dose-dependently reduced CRC, but the relative importance of uncoupling versus volume changes on CRC differed between brain and heart mitochondria (data to be published elsewhere).
DISCUSSION
The main finding of the present study was that increased K+ conductance, modeled by low concentrations of valinomycin, increases mitochondrial CRC and resistance to mPT in isolated brain mitochondria. Furthermore, CRC was improved by reducing the osmolarity of the surrounding buffer, indicating that the beneficial effect of increased K+ conductance could be attributed to a direct physiological effect of an enhanced mitochondrial matrix volume.
How Increased K+ Conductance May Improve Mitochondrial Calcium Retention and Sensitivity to Permeability Transition
Increasing mitochondrial K+ conductance by valinomycin or by activation of a K+ channel (such as the putative mitoKATP) will cause K+ to enter mitochondria along its electrochemical gradient. The drop in membrane potential will accelerate NADH oxidation at complex I to rebuild the proton motive force. Increased activity of the K+/H+ exchanger will partially counteract the influx of K+, and depending on the relative increase of K+ conductance versus K+ extrusion, a new equilibrium will be reached. The previously proposed physiological effects of this process, besides a reduction in ΔΨm and increased respiration, are (i) matrix alkalization due to the K+/H+ exchange, (ii) increased mitochondrial ROS production, which in turn may activate endogenous protective pathways, and (iii) matrix swelling caused by the osmotic effect of K+ (22, 44) (Fig. 4).
Matrix pH and Calcium Retention
Mitochondria take up calcium electrophoretically through the uniporter above a certain external [Ca2+], the so-called calcium set-point (40). The calcium uptake capacity of mitochondria is very large in the presence of physiological levels of ADP and Pi, but the intramitochondrial free [Ca2+] is kept low because of formation of inactive calcium phosphates, e.g. Ca3(PO4)2. The complexation of calcium requires PO43−, and both the uptake of H2PO4− and the successive deprotonations to PO43− vary with matrix pH. The free [Ca2+] in equilibrium with Ca3(PO4)2 would, thus, vary significantly in relation to matrix pH due to Pi availability. A theoretic example for [Ca2+] in equilibrium with Ca3(PO4)2 in the presence of 2 mm Pi has been presented by D. G. Nicholls (40), where [Ca2+] would increase from 2 to 100 μm if the matrix pH was reduced from 7.8 to 7.0. Thus, a low matrix pH and Pi content will have a dramatic effect on calcium-phosphate formation, and free matrix [Ca2+] may be elevated to an extent where mPT activation will occur. Our results demonstrate that an expected reduced ΔpH, i.e. decreased matrix pH, resulted in a reduced CRC regardless if it occurred concomitantly to an increased ΔΨm (as with nigericin) or a decreased ΔΨm (as with CCCP). Furthermore, when external pH was manipulated in mitochondria with or without nigericin treatment, an alkaline matrix pH (up to a certain extent) was coupled to a high CRC, whereas an acidic to neutral matrix pH resulted in low CRC. In de-energized mitochondria, where respiration and consequently ΔΨm-dependent calcium transport is inhibited and/or calcium transport is facilitated by a calcium-ionophore, low pH is a potent inhibitor, and Pi is a main inducer of mPT (37, 38, 45), and this is the general view of their role in mPT regulation (46, 47). We demonstrate using high resolution respirometry during calcium infusion that mPT is the end point of mitochondrial calcium retention. The determination of CRC and the simultaneous monitoring of O2 flux offer reliable and quantitative assays for mPT in a physiological context where both negative and positive effects of a given treatment are taken into account. The strong pH dependence of brain mitochondria to buffer external calcium loads demonstrate that the effects on free matrix [Ca2+] by pH and Pi during active mitochondrial calcium loading are more important than their effect on the mPT pore components per se.
Several previous studies using isolated mitochondria have demonstrated that valinomycin can increase matrix pH (22, 44, 48, 49). On the other hand, in a physiological medium, permeant anions such as Pi, which are translocated into mitochondria depending on ΔpH, may buffer a matrix pH elevation induced by K+ flux (48). Pi may also be the main charge-compensating anion accompanying mitochondrial K+ uptake as the mitochondrial matrix volume increases. Indeed, we demonstrate here in equilibrium distribution measurements of tritiated acetate using a Pi, substrate, and adenine nucleotide-containing buffer, that no change in ΔpH or calcium set-point occurs after valinomycin treatment. Thus, increased K+ conductance did not mediate its beneficial effect on CRC through an augmented ΔpH.
Mitochondrial ROS Generation and PKCϵ Activation
Activation of PKCϵ is considered to be a key mediator of ischemic preconditioning in the heart (50, 51). Besides a role in activating mitoKATP (52), PKCϵ has been shown to associate with and inhibit activation of the mPT pore complex (53). Increased mitochondrial K+ conductance has been suggested to increase mitochondrial ROS production both in vitro and in vivo (44, 54). PKCϵ is believed to be activated by the elevated ROS, desensitizing mPT to activation by calcium (23). The increased ROS has been proposed to be a direct effect of mitoKATP- or valinomycin-induced alkalization of matrix pH (44). However, in our hands valinomycin consistently decreased detected H2O2 in a dose-dependent fashion, and the K+ carrier significantly lowered the generation of H2O2 during calcium infusion, likely related to the depolarization induced by the compound. Furthermore, inhibition of PKCϵ and H2O2 scavenging did not inhibit the beneficial effect of valinomycin. Thus, we conclude that this pathway does not seem to be active in our mitochondrial preparations and does not underlie the beneficial effect of increased K+ conductance.
Matrix Volume and Calcium Retention
In contrast, increasing matrix volume by lowering the KCl concentration of the surrounding medium resulted in an enhanced CRC similar to that induced by valinomycin, demonstrating that the beneficial effect of increased K+ conductance in brain mitochondria is probably mediated by an enhanced matrix volume. Respiration and energy transfer can be modulated by matrix volume (25, 26), but no difference in respiration was observed in mitochondria suspended in 75 or 125 mm KCl. Extramitochondrial osmolarity has been shown to influence the sensitivity of liver mitochondria to mPT, which was attributed to volume-dependent concentrations of endogenous mPT modulators (55). Changes in matrix volume have also been demonstrated to alter the mitochondrial affinity for ADP, where an increased matrix volume was associated with reduced ADP affinity (56), but this mechanism would rather sensitize mitochondria to mPT than increase CRC. The expanded matrix volume may, however, hold more Pi and reduce some physicochemical restraints in the protein dense matrix to facilitate calcium complex formation.
Mild Uncoupling and Calcium Retention
Mild uncoupling of mitochondria, i.e. slightly reducing the proton motive force and ΔΨm by increasing H+ conductance by, e.g. protonophores, has been suggested to be a beneficial strategy against mitochondrial-mediated cell damage via decreased ROS generation and limitation of mitochondrial calcium uptake (57, 58). Diazoxide has been shown to concentration-dependently (IC50 65 μm) decrease the rate and magnitude of calcium uptake in isolated heart mitochondria (58). However, the authors further reported that diazoxide activated mitochondrial release of calcium, a process that was prevented by cyclosporin A. This indicates that diazoxide at high concentrations actually sensitized the mitochondria toward mPT and the associated calcium release (which we also demonstrate in the present study) rather than afforded protection against mPT. It is likely that these effects of diazoxide are related to unspecific respiratory inhibition and depolarization at high concentrations rather than specific activity of mitoKATP. A hampered mitochondrial function caused by high dosing of diazoxide or other pharmacological agents secondarily reducing calcium uptake and retention are likely not compatible with the high energy demand of neuronal cells, in particular during pathological situations of increased metabolic stress and cytoplasmic calcium overload (59). Mimicking K+ channel activity with valinomycin demonstrated an opposite effect to diazoxide (and CCCP) on CRC in our hands. We suggest that if mild uncoupling is beneficial, increased K+ conductance rather than increased H+ conductance may be a safer approach to reduce ROS as it would not pose the threat of sensitizing mitochondria to mPT during calcium accumulation.
MitoKATP
Several lines of evidence indicate the existence of mitoKATP and a prominent role of these channels in the preconditioning effect of cardiac and cerebral ischemia (7, 8, 12). Several authors have, however, failed to detect the existence of mitoKATP in isolated heart and brain mitochondria (32, 33), and the specificity of diazoxide and 5-hydroxydecanoate has been questioned (43, 60). Lack of specific effects of diazoxide could indicate that mitoKATP are not present in mitochondria and that the pharmacological effects of diazoxide and other putative ligands of this channel are caused by interaction with other pharmacological or unspecific targets. In line with this is the suggestion that ischemic and pharmacological preconditioning can induce protection through nonspecific effects on mitochondrial function including respiratory chain inhibition and ROS release from the mitochondria (21). Another alternative is that the channel is inactivated during isolation of mitochondria or that a cofactor needs to be present, in which cases the variable findings can depend on minor differences in mitochondrial isolation procedures. Although the existence of mitoKATP may be controversial, electrophoretic K+ flux in mitochondria is well established (26, 46, 61), and the concept of altered K+ flux of the inner mitochondrial membrane in preconditioning remains thrilling. A Kv1.3 and a Ca2+-activated K+ channel are other candidates for mitochondrial K+ channels relevant for preconditioning, and ligands of the latter demonstrate a similar cardioprotective effect as ligands of mitoKATP (60, 62–64).
Clinical Perspective
Inhibition of cyclophilin D, thereby reducing the sensitivity to mPT, is a potential strategy for neuroprotection in acute and chronic neurodegenerative disease as well as for cardioprotection in ischemia-reperfusion injury. The cyclophilin D inhibitor cyclosporin A has displayed promising results in a human clinical trial of myocardial reperfusion injury (65) and human clinical trials in traumatic brain injury (66–68). The findings of the present study indicate that activation of mitochondrial K+ channels could provide an additional means of improving mitochondrial resistance to calcium overload and activation of mPT through an enhanced matrix volume. Augmenting the brain mitochondrial ability to retain calcium by matrix volume modulation may, thus, be an end-effector of preconditioning and ischemic tolerance and could constitute a novel target for therapeutic intervention.
Acknowledgments
We are grateful to David Nicholls, Andrew Halestrap, and Carsten Ruscher for methodological input, to Tadeusz Wieloch for support, and to Fredrik Leeb-Lundberg for use of scintillation counter. Debio-025 (D-MeAla3EtVal4-cyclosporin) was kindly provided by Debiopharm S.A.
This work was supported by the Swedish Research Council (reference number 2008-2634), by the Japanese Ministry of Health and Labor and Welfare Grant 18591724, by the Swedish Society of Medicine, and by the foundations of Stohne, Segerfalk, and the Royal Physiographic Society in Lund. E. Elmér is co-founder and officer of Maas Biolab, LLC and NeuroVive Pharmaceutical AB, which hold intellectual property rights and develop the use of cyclosporins as cyclophilin D inhibitors for neurological treatment.
- mitoKATP
- mitochondrial ATP-sensitive potassium channels
- mPT
- mitochondrial permeability transition
- CRC
- calcium retention capacity
- Debio-025 (UNIL025)
- D-MeAla3EtVal4-cyclosporin
- ΔΨm
- mitochondrial membrane potential
- PKCϵ
- protein kinase Cϵ
- ROS
- reactive oxygen species
- MPG
- N-2-mercaptopropionylglycine
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone.
REFERENCES
- 1.Janoff A. (1964) Int. Anesthesiol. Clin. 2, 251–269 [DOI] [PubMed] [Google Scholar]
- 2.Murry C. E., Jennings R. B., Reimer K. A. (1986) Circulation 74, 1124–1136 [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa K., Matsumoto M., Tagaya M., Hata R., Ueda H., Niinobe M., Handa N., Fukunaga R., Kimura K., Mikoshiba K., et al. (1990) Brain Res 528, 21–24 [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U., Simon R. P., Hallenbeck J. M. (2003) Trends Neurosci. 26, 248–254 [DOI] [PubMed] [Google Scholar]
- 5.Chopp M., Chen H., Ho K. L., Dereski M. O., Brown E., Hetzel F. W., Welch K. M. (1989) Neurology 39, 1396–1398 [DOI] [PubMed] [Google Scholar]
- 6.Gidday J. M. (2006) Nat. Rev. Neurosci. 7, 437–448 [DOI] [PubMed] [Google Scholar]
- 7.Yellon D. M., Downey J. M. (2003) Physiol. Rev. 83, 1113–1151 [DOI] [PubMed] [Google Scholar]
- 8.Ardehali H., O'Rourke B. (2005) J. Mol. Cell. Cardiol. 39, 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlid K. D., Paucek P., Yarov-Yarovoy V., Sun X., Schindler P. A. (1996) J. Biol. Chem. 271, 8796–8799 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Sato T., O'Rourke B., Marban E. (1998) Circulation 97, 2463–2469 [DOI] [PubMed] [Google Scholar]
- 11.Jabùrek M., Yarov-Yarovoy V., Paucek P., Garlid K. D. (1998) J. Biol. Chem. 273, 13578–13582 [PubMed] [Google Scholar]
- 12.Liu D., Lu C., Wan R., Auyeung W. W., Mattson M. P. (2002) J. Cereb. Blood Flow Metab. 22, 431–443 [DOI] [PubMed] [Google Scholar]
- 13.Domoki F., Perciaccante J. V., Veltkamp R., Bari F., Busija D. W. (1999) Stroke 30, 2713–2719 [DOI] [PubMed] [Google Scholar]
- 14.Halestrap A. P. (2009) J. Bioenerg. Biomembr. 41, 113–121 [DOI] [PubMed] [Google Scholar]
- 15.Bernardi P., Krauskopf A., Basso E., Petronilli V., Blachly-Dyson E., Di Lisa F., Forte M. A. (2006) FEBS J. 273, 2077–2099 [DOI] [PubMed] [Google Scholar]
- 16.Crompton M. (1999) Biochem. J. 341, 233–249 [PMC free article] [PubMed] [Google Scholar]
- 17.Wieloch T., Mattiasson G., Hansson M. J., Elmér E. (2007) in Handbook of Neurochemistry and Molecular Neurobiology-Brain Energetics. Integration of Molecular and Cellular Processes (Lajtha A., Gibson G. E., Dienel G. A. eds) 3rd Ed., pp. 667–702, Springer, New York, NY [Google Scholar]
- 18.Forte M., Gold B. G., Marracci G., Chaudhary P., Basso E., Johnsen D., Yu X., Fowlkes J., Rahder M., Stem K., Bernardi P., Bourdette D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7558–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schinzel A. C., Takeuchi O., Huang Z., Fisher J. K., Zhou Z., Rubens J., Hetz C., Danial N. N., Moskowitz M. A., Korsmeyer S. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12005–12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hausenloy D. J., Maddock H. L., Baxter G. F., Yellon D. M. (2002) Cardiovasc Res 55, 534–543 [DOI] [PubMed] [Google Scholar]
- 21.Hausenloy D. J., Yellon D. M., Mani-Babu S., Duchen M. R. (2004) Am. J. Physiol. Heart Circ. Physiol. 287, H841–H849 [DOI] [PubMed] [Google Scholar]
- 22.Costa A. D., Quinlan C. L., Andrukhiv A., West I. C., Jabùrek M., Garlid K. D. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H406–H415 [DOI] [PubMed] [Google Scholar]
- 23.Costa A. D., Jakob R., Costa C. L., Andrukhiv K., West I. C., Garlid K. D. (2006) J. Biol. Chem. 281, 20801–20808 [DOI] [PubMed] [Google Scholar]
- 24.Chalmers S., Nicholls D. G. (2003) J. Biol. Chem. 278, 19062–19070 [DOI] [PubMed] [Google Scholar]
- 25.Nicholls D. G., Lindberg O. (1972) FEBS Lett. 25, 61–64 [DOI] [PubMed] [Google Scholar]
- 26.Halestrap A. P. (1989) Biochim. Biophys. Acta 973, 355–382 [DOI] [PubMed] [Google Scholar]
- 27.Costa A. D., Garlid K. D. (2009) J. Bioenerg. Biomembr. 41, 123–126 [DOI] [PubMed] [Google Scholar]
- 28.Sims N. R., Anderson M. F. (2008) Nat. Protoc. 3, 1228–1239 [DOI] [PubMed] [Google Scholar]
- 29.Hansson M. J., Månsson R., Mattiasson G., Ohlsson J., Karlsson J., Keep M. F., Elmér E. (2004) J. Neurochem. 89, 715–729 [DOI] [PubMed] [Google Scholar]
- 30.Hütter E., Unterluggauer H., Garedew A., Jansen-Dürr P., Gnaiger E. (2006) Exp. Gerontol. 41, 103–109 [DOI] [PubMed] [Google Scholar]
- 31.Nicholls D. G. (1974) Eur. J. Biochem. 50, 305–315 [DOI] [PubMed] [Google Scholar]
- 32.Das M., Parker J. E., Halestrap A. P. (2003) J. Physiol. 547, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brustovetsky T., Shalbuyeva N., Brustovetsky N. (2005) J. Physiol. 568, 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. (1967) Proc. Natl. Acad. Sci. U.S.A. 58, 1949–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristian T., Pivovarova N. B., Fiskum G., Andrews S. B. (2007) J. Neurochem. 102, 1346–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petronilli V., Nicolli A., Costantini P., Colonna R., Bernardi P. (1994) Biochim. Biophys. Acta 1187, 255–259 [DOI] [PubMed] [Google Scholar]
- 37.Halestrap A. P. (1991) Biochem. J. 278, 715–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haworth R. A., Hunter D. R. (1979) Arch Biochem. Biophys. 195, 460–467 [DOI] [PubMed] [Google Scholar]
- 39.Kristian T., Bernardi P., Siesjö B. K. (2001) J Neurotrauma 18, 1059–1074 [DOI] [PubMed] [Google Scholar]
- 40.Nicholls D. G. (2005) Cell Calcium 38, 311–317 [DOI] [PubMed] [Google Scholar]
- 41.Baysal K., Jung D. W., Gunter K. K., Gunter T. E., Brierley G. P. (1994) Am. J. Physiol. 266, C800–C808 [DOI] [PubMed] [Google Scholar]
- 42.Zoccarato F., Nicholls D. (1982) Eur. J. Biochem. 127, 333–338 [DOI] [PubMed] [Google Scholar]
- 43.Dröse S., Brandt U., Hanley P. J. (2006) J. Biol. Chem. 281, 23733–23739 [DOI] [PubMed] [Google Scholar]
- 44.Andrukhiv A., Costa A. D., West I. C., Garlid K. D. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H2067–H2074 [DOI] [PubMed] [Google Scholar]
- 45.Bernardi P., Vassanelli S., Veronese P., Colonna R., Szabó I., Zoratti M. (1992) J. Biol. Chem. 267, 2934–2939 [PubMed] [Google Scholar]
- 46.Bernardi P. (1999) Physiol. Rev. 79, 1127–1155 [DOI] [PubMed] [Google Scholar]
- 47.Zoratti M., Szabò I. (1995) Biochim. Biophys. Acta 1241, 139–176 [DOI] [PubMed] [Google Scholar]
- 48.Dodgson S. J., Forster R. E., 2nd, Storey B. T. (1982) J. Biol. Chem. 257, 1705–1711 [PubMed] [Google Scholar]
- 49.Hutson S. M. (1987) J. Biol. Chem. 262, 9629–9635 [PubMed] [Google Scholar]
- 50.Ping P., Song C., Zhang J., Guo Y., Cao X., Li R. C., Wu W., Vondriska T. M., Pass J. M., Tang X. L., Pierce W. M., Bolli R. (2002) J. Clin. Invest. 109, 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saurin A. T., Pennington D. J., Raat N. J., Latchman D. S., Owen M. J., Marber M. S. (2002) Cardiovasc. Res. 55, 672–680 [DOI] [PubMed] [Google Scholar]
- 52.Jabùrek M., Costa A. D., Burton J. R., Costa C. L., Garlid K. D. (2006) Circ. Res. 99, 878–883 [DOI] [PubMed] [Google Scholar]
- 53.Baines C. P., Song C. X., Zheng Y. T., Wang G. W., Zhang J., Wang O. L., Guo Y., Bolli R., Cardwell E. M., Ping P. (2003) Circ. Res. 92, 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pain T., Yang X. M., Critz S. D., Yue Y., Nakano A., Liu G. S., Heusch G., Cohen M. V., Downey J. M. (2000) Circ. Res. 87, 460–466 [DOI] [PubMed] [Google Scholar]
- 55.Nogueira V., Devin A., Walter L., Rigoulet M., Leverve X., Fontaine E. (2005) J. Bioenerg. Biomembr. 37, 25–33 [DOI] [PubMed] [Google Scholar]
- 56.Dos Santos P., Kowaltowski A. J., Laclau M. N., Seetharaman S., Paucek P., Boudina S., Thambo J. B., Tariosse L., Garlid K. D. (2002) Am. J. Physiol. Heart Circ. Physiol. 283, H284–H295 [DOI] [PubMed] [Google Scholar]
- 57.Brand M. D., Affourtit C., Esteves T. C., Green K., Lambert A. J., Miwa S., Pakay J. L., Parker N. (2004) Free Radic. Biol. Med. 37, 755–767 [DOI] [PubMed] [Google Scholar]
- 58.Holmuhamedov E. L., Wang L., Terzic A. (1999) J. Physiol. 519, 347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morota S., Månsson R., Hansson M. J., Kasuya K., Shimazu M., Hasegawa E., Yanagi S., Omi A., Uchino H., Elmér E. (2009) Exp Neurol. 218, 353–362 [DOI] [PubMed] [Google Scholar]
- 60.Hanley P. J., Daut J. (2005) J. Mol. Cell. Cardiol. 39, 17–50 [DOI] [PubMed] [Google Scholar]
- 61.Brierley G. P., Baysal K., Jung D. W. (1994) J. Bioenerg. Biomembr. 26, 519–526 [DOI] [PubMed] [Google Scholar]
- 62.Siemen D., Loupatatzis C., Borecky J., Gulbins E., Lang F. (1999) Biochem. Biophys. Res. Commun. 257, 549–554 [DOI] [PubMed] [Google Scholar]
- 63.Szabò I., Bock J., Jekle A., Soddemann M., Adams C., Lang F., Zoratti M., Gulbins E. (2005) J. Biol. Chem. 280, 12790–12798 [DOI] [PubMed] [Google Scholar]
- 64.Xu W., Liu Y., Wang S., McDonald T., Van Eyk J. E., Sidor A., O'Rourke B. (2002) Science 298, 1029–1033 [DOI] [PubMed] [Google Scholar]
- 65.Piot C., Croisille P., Staat P., Thibault H., Rioufol G., Mewton N., Elbelghiti R., Cung T. T., Bonnefoy E., Angoulvant D., Macia C., Raczka F., Sportouch C., Gahide G., Finet G., André-Fouët X., Revel D., Kirkorian G., Monassier J. P., Derumeaux G., Ovize M. (2008) N. Engl. J. Med. 359, 473–481 [DOI] [PubMed] [Google Scholar]
- 66.Empey P. E., McNamara P. J., Young B., Rosbolt M. B., Hatton J. (2006) J. Neurotrauma 23, 109–116 [DOI] [PubMed] [Google Scholar]
- 67.Mazzeo A. T., Alves O. L., Gilman C. B., Hayes R. L., Tolias C., Niki Kunene K., Ross Bullock M. (2008) Acta Neurochir. (Wien) 150, 1019–1031 [DOI] [PubMed] [Google Scholar]
- 68.Hatton J., Rosbolt B., Empey P., Kryscio R., Young B. (2008) J. Neurosurg. 109, 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]