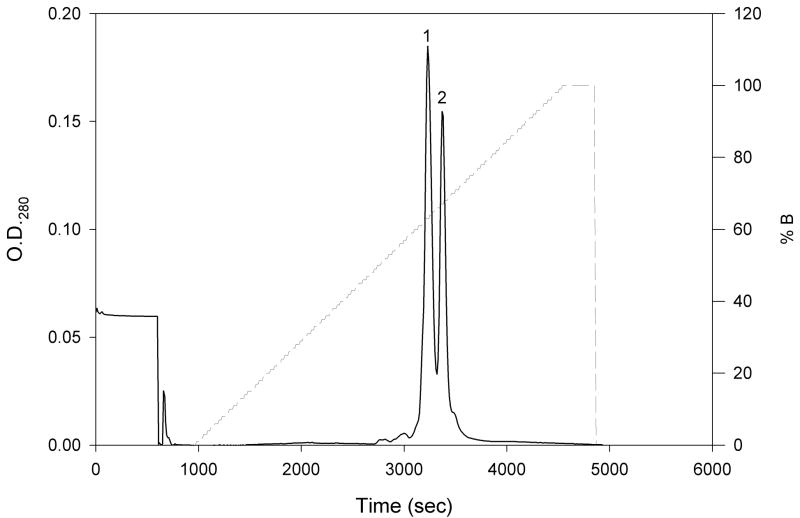
Figure 2.
Chromatogram of the separation of N-TIMP-1 by high-resolution cation exchange chromatography. N-TIMP-1 (10 mg) purified by cation exchange chromatography with CM-52 was loaded onto a new Mono S HR 5/5 cation exchange column pre-equilibrated with buffer A (20 mM Bis Tris-HCl, pH 5.5). The column was then washed with 10 mL buffer A, and bound protein was eluted with a linear gradient of 0–100% buffer B (20 mM Bis Tris-HCl, pH 5.5, 0.5 M NaCl) over 60 minutes at a flow rate of 1mL/min. The two components are labeled peaks 1 and 2, respectively.