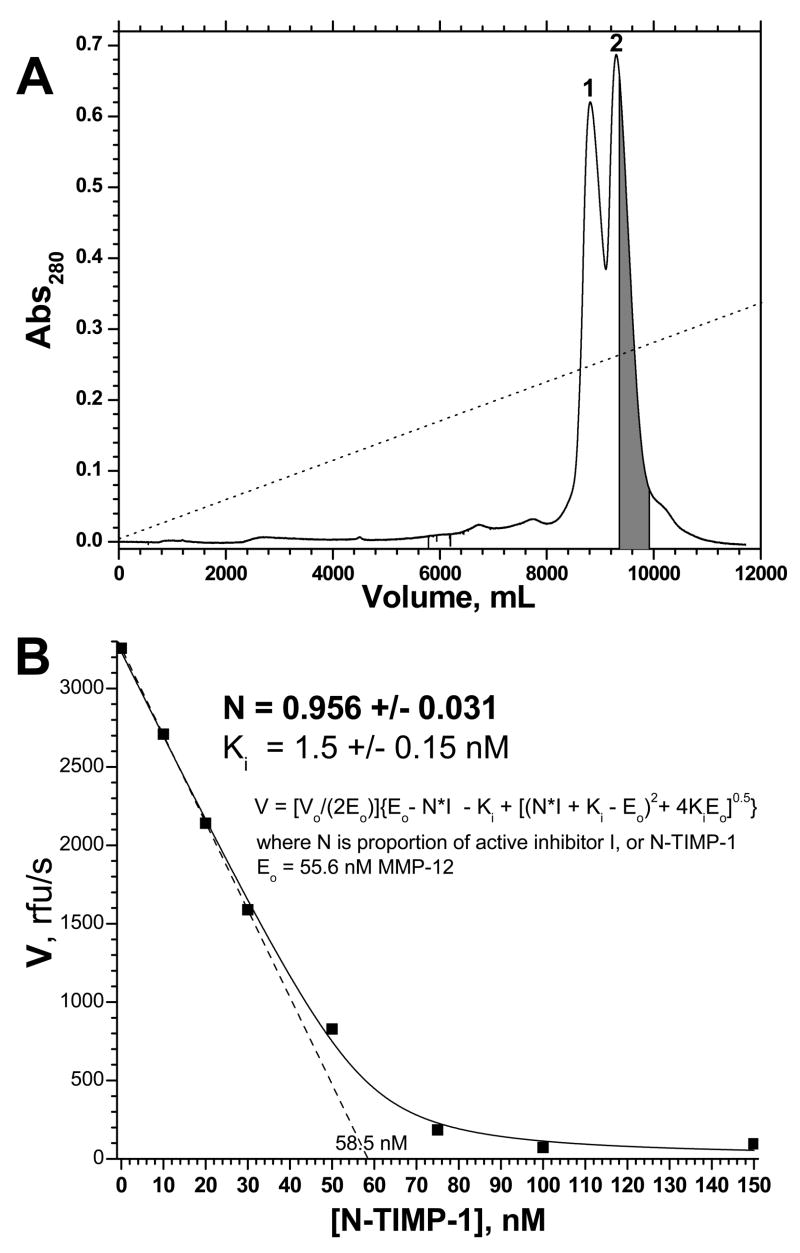
Figure 4.
Purification of N-TIMP-1 by cation exchange chromatography (A) and demonstration of its high purity and activity by titration of MMP-12(ΔC) (B). Panel (A): The separation was performed similarly as in Fig. 2, except that the Mono S HR 5/5 cation exchange had experienced normal, repeated usage and that the gradient was lengthened to 240 min and the maximum [NaCl] was decreased to 0.25 M. The portion of peak 2 marked gray was collected for active site titration. Panel (B): The gray portion of peak 2 was titrated into MMP-12(ΔC) having 55.6 nM intact active sites by titration with GM6001. MMP-12(ΔC)’s initial velocities as a function of total, purified [N-TIMP-1] were fitted to the slightly modified Morrison tight-binding inhibition equation shown (eq. 1), in order to determine N, the proportion of the inhibitor N-TIMP-1 that is active, along with Ki. The uncertainties listed are the fitting errors. Experimental uncertainties may be 5% or more.