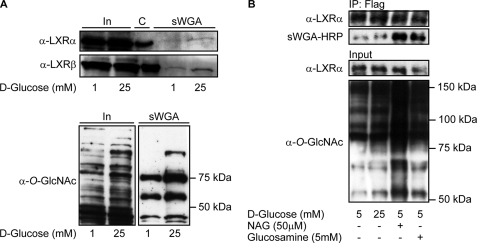
FIGURE 3.
LXR GlcNAcylation is elevated by glucose in Huh7 cells and in stably transfected FLAG-hLXRα FlpInTM293 cells. A, GlcNAc-modified proteins from FLAG-hLXRα or FLAG-hLXRβ-overexpressing Huh7 cells (1 mm and 25 mm glucose) were absorbed on sWGA-agarose beads (total amount of proteins loaded onto sWGA beads was the same in all lanes). Input (In, 10%) and sWGA-precipitated proteins (sWGA) were subjected to SDS-PAGE and blotted using anti-LXRα or anti-LXRβ antibodies. Immunoprecipitated LXRs are loaded as size control (C) (upper panel). O-GlcNAc-modified proteins from input (10%, left panel) and sWGA precipitation (right panel) were detected using anti-O-GlcNAc (CTD110.6) antibody (lower panel). B, FLAG-hLXRα was immunoprecipitated (IP) from FLAG-hLXRα stably transfected FlpInTM293 cells (5 mm glucose, 25 mm glucose, 5 mm glucose + GlcNAc (NAG), 5 mm glucose + glucosamine) using rabbit anti-FLAG antibody. Input (2%) and immunoprecipitated proteins were subjected to SDS-PAGE and blotted with anti-LXRα antibody and sWGA-HRP or anti-O-GlcNAc (CTD110.6) antibody to verify GlcNAc modification.