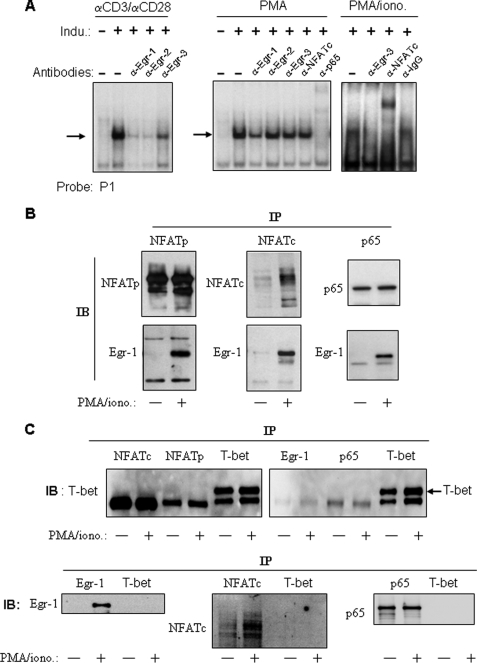
FIGURE 3.
Egr-1 binds to the IL-4 P1 enhancer element and interacts with NFAT and NF-κB. A, Egr-1 binds to the human IL-4 P1 enhancer element upon stimulation with PMA or α-CD3/α-CD28. The 32P-labeled IL-4 P1 probe was incubated with nuclear extracts (10 μg) prepared from Jurkat T cells nonstimulated (−) or stimulated (+) for 2 h with α-CD3 (30 μg/ml)/α-CD28 (5 μg/ml), PMA (10 ng/ml), or PMA/ionomycin (1 μm) (PMA/iono.) in the absence or presence of the indicated antibodies. After 30 min of incubation, the samples were subjected to EMSA. The inducible complexes are indicated by arrows. Results are representative of two independent experiments. B, Egr-1 co-immunoprecipitates with NFAT and NF-κB. Total lysates from Jurkat cells either nonstimulated (−) or stimulated (+) with PMA/ionomycin for 2 h were immunoprecipitated (IP) with antibodies against NFATp, NFATc, or NF-κB subunit p65 and then immunoblotted (IB) with α-Egr-1 antibodies or the indicated control antibodies. Results are representative of three independent experiments. C, unrelated antibody, α-T-bet, was used as control and showed that NFAT, p65, and Egr-1 did not co-precipitate with T-bet.