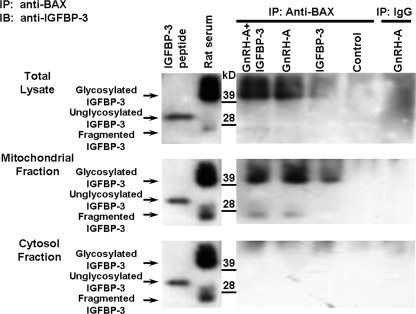
FIGURE 1.
Endogenous IGFBP-3 interacts with BAX in the rat testis. Western blots for IGFBP-3 after IP of the testis homogenates with anti-BAX or IgG (negative control for the IP step) are shown. Rats were treated with vehicle (Control), GnRH-A, IGFBP-3 peptide, or GnRH-A plus IGFBP-3 peptide, as described under “Experimental Procedures.” Anti-BAX antibody was used to immunoprecipitate IGFBP-3. Synthetic IGFBP-3 peptide was used as positive control for unglycosylated IGFBP-3 (molecular mass ∼30 kDa), and rat serum was shown as positive control for glycosylated IGFBP-3 (molecular mass ∼40–42 kDa) (right panels). IGFBP-3 fragments (molecular mass ∼ 20–24 kDa) were detected only in serum and mitochondrial fractions after GNRH-A treatments. Endogenous IGFBP-3 expression was not detected after IP with rabbit IgG in GnRH-A-treated testis tissue (IP-IgG, right lane, negative control for IP). After co-immunoprecipitation with anti-BAX antibody, endogenous IGFBP-3 (glycosylated IGFBP-3, molecular mass ∼40–42 kDa) was detectable by Western blotting with anti-IGFBP-3 after in vivo treatment with GnRH-A with or without IGFBP-3 in testis total lysate (top panel) or mitochondrial fraction (middle panel), but not detected in the control group. Endogenous IGFBP-3 was not detectable in testis cytosol fraction (bottom panel) in control and all treated animal groups.