FIGURE 1.
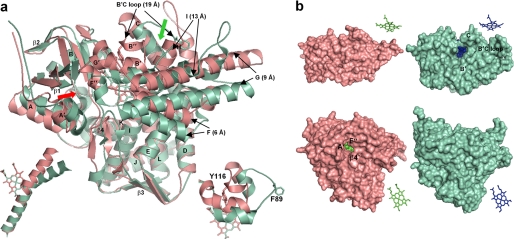
Ligand-free Tbb14DM (salmon). a, superposition with MtCYP51 (1e9x) (green), ribbon diagram. The heme, Tyr-116 (Tbb), and Phe-89 (Mt) are shown in stick representation. The most divergent active site-defining regions are indicated with black arrows, and distances between the equivalent Cα atoms are provided in parentheses. The locations of the putative substrate entrance in Tbb and Mt structures are shown with red and green arrows, respectively. The structure of ligand-free MtCYP51 (1h5z) is identical to 1e9x except for its larger opening to the upper surface due to missing density for helix C. Left inset, I-helix in Tbb and Mt CYP51, distal view. Right inset, BC loop, upper view. b, surface representations of Tbb and Mt CYP51 from the upper and distal sides; the corresponding heme orientation is shown on the right. Inside the proteins, the heme is presented as spheres. In Tbb14DM, the channel extends about 20 Å from the distal surface to the iron, forming an angle of about 50° to the heme plane. In MTCYP51, the funnel-like opening runs from above perpendicular to the heme plane.