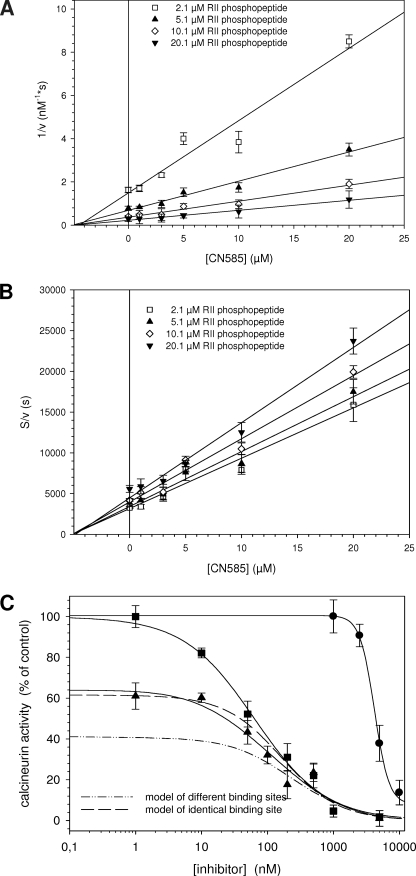
FIGURE 4.
Analysis of the inhibition type and putative calcineurin binding site. Inhibition type of CN585 was evaluated using substrate concentration from 2.1 to 20.1 μm RII phosphopeptide mixture containing 100 nm biotinylated and 0.5–20 μm nonbiotinylated RII phosphopeptide. The inhibition mode and the Ki value of CN585 were determined by plotting 1/v against CN585 (Dixon plot) (A) and by plotting [S]/v against CN585 (B). C, to obtain indications about the CN585 binding site on calcineurin, the phosphatase was simultaneously inhibited with CN585 and increasing concentrations of the Cyp18·CsA complex. Measurements were performed using 33P-labeled RII phosphopeptide, 3 μm CN585, 10 μm CsA, and various Cyp18 concentrations. Because of the high affinity between Cyp18 and CsA, the concentration of the Cyp18·CsA complex is identical to the applied Cyp18 concentration. Moreover, by using DynaFit software, two theoretical binding curves were calculated according to a model of identical binding sites (long dash) and a model of different binding sites (dash-dot-dot) of CN585 and Cyp18·CsA. The shape of the measured curves (solid line) clearly indicates a binding on identical or overlapping binding sites of both inhibitors. Moreover, the concentration-dependent inhibition of calcineurin by Cyp18·CsA and CN585 alone is also displayed in the figure. All experimental data presented are the means ± S.D. of three independent experiments.