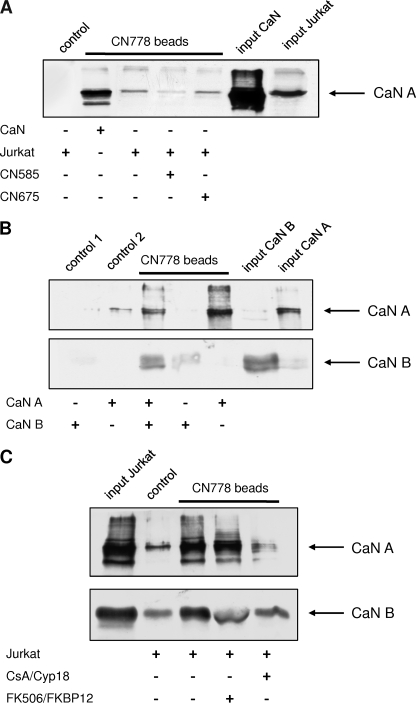
FIGURE 5.
Coprecipitation of recombinant and endogenous calcineurin using CN778 affinity matrix. A, biotinylated CN585 derivative, CN778, was immobilized onto streptavidin-Sepharose. Beads were incubated with recombinant calcineurin or Jurkat cell lysate in the presence and absence of CN585 or CN675 for 3 h at 4 °C. Recombinant calcineurin (CaN) and endogenous calcineurin of Jurkat cells interacts with the affinity matrix. CN585 interferes with calcineurin binding, whereas the non-inhibitory compound CN675 has no effect on calcineurin interaction. B, binding of calcineurin subunits onto the CN778 matrix is shown. The catalytic subunit of calcineurin A (CaN A) and the regulatory subunit B (CaN B) were incubated separately or together with the immobilized CN778. The regulatory subunit shows no interaction with the inhibitor, whereas the catalytic subunit interacts with the inhibitor matrix. C, shown is competition of calcineurin interaction to CN585 by CsA·Cyp18 and FK506·FKBP12 complexes. CN778 affinity matrix was incubated with Jurkat cell lysate, and after several washing steps, CsA·Cyp18 and FK506·FKBP12 complexes were added. In the case of CsA·Cyp18, calcineurin binding to the beads was strongly diminished, whereas the FK506·FKBP12 had no effect on calcineurin interaction. Biotin-saturated streptavidin-Sepharose was used as control. Subsequently, the matrix was washed with PBS and boiled in SDS sample buffer, and bound proteins were separated by SDS-PAGE. The proteins were blotted onto nitrocellulose membrane, probed with anti-calcineurin antibody, and analyzed by ECL reaction.