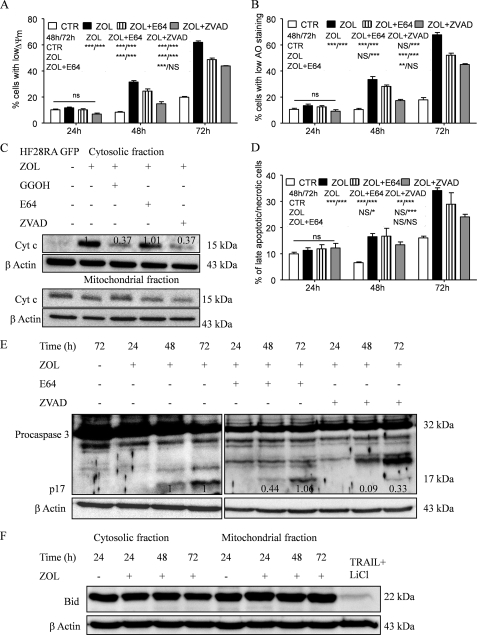
FIGURE 6.
Effect of caspase/cathepsin inhibitors on ZOL-induced apoptosis in HF28RA cells. Inhibition of ZOL-induced apoptosis of the follicular lymphoma cell line was estimated in co-culture with the pancaspase inhibitor z-VAD and the cathepsin B/L inhibitor, E64. Cells were preincubated (2 h) with each inhibitor (60 μm for z-VAD-fmk, 100 μm for E64) and then exposed for up to 72 h to 50 μm ZOL. z-VAD-fmk and E64 alone did not have any effect on the proliferation of HF28RA cells (data not shown). Detection of Δψm reduction (A), lysosomal membrane permeabilization (B), and cell membrane integrity (D) was measured by fluorescence-activated cell sorter analysis. Statistical evaluation of the data were performed using the ANOVA test; NS, not significant (p > 0.05); p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) when compared with control and ZOL and ZOL+E64-treated cells versus corresponding treatment effects, as depicted in the data underneath the legends, on MMP, LMP, and membrane integrity, respectively. CTL, control. C, effects of caspase/cathepsin inhibitors and GGOH on ZOL-induced cytochrome c (Cyt c) release in HF28RA cells. Cells were preincubated (2 h) with caspase/cathepsin inhibitor (60 μm for z-VAD-FMK, 100 μm for E64) or directly co-incubated with geranylgeranyl (10 μm GGOH) before being exposed to 50 μm ZOL for 72 h. Cytochrome c expression was monitored by Western blot and followed by densitometric analysis normalized by the internal control (β-actin) and relative to the respective cytochrome c level from cells exposed to ZOL alone for 72 h, which was set to 1. E, shown is a Western blot analysis of caspase-3 cleavage. Equal loading was controlled by immunodetection of β-actin. Numbers underneath the bands represent densitometric analysis normalized to β-actin and relative to the corresponding levels of the 17-kDa caspase-3-activated fragment from ZOL-alone-treated cells, which was set to 1. F, time course of full-length Bid expression (22 kDa) in HF28RA GFP cells treated with 50 μm ZOL. Equal amounts of protein (20 μg) obtained after fractionation of cytosolic and mitochondrial fractions were separated by SDS-PAGE on 15% gels. β-Actin was used as a loading control. Bid was detected as a 22-kDa protein. As a positive control we used an extract from TRAIL + LiCl-treated HF28RA wild type cells where full-length Bid expression decreased. Two independent experiments yielded comparable results.