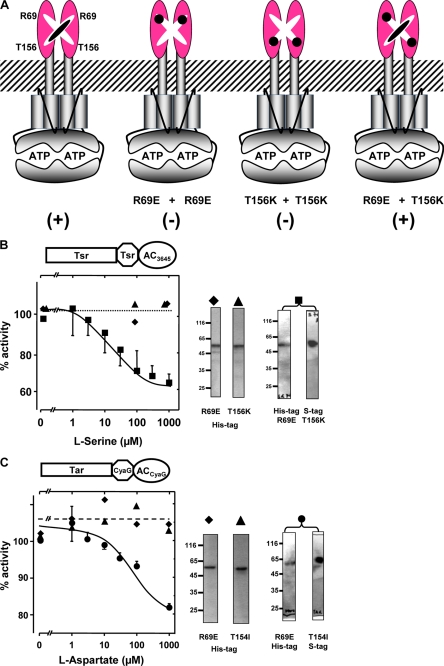
FIGURE 6.
Functional complementation of inactivated Tsr and Tar receptor point mutants. A, the rationale for receptor complementation is shown. The numbering refers to Tsr. Neither R69E nor T156K mutant dimers bind ligand. In heterodimers (right) a serine binding site is reconstituted. B, a complementation assay of Tsr AC Rv3645 mutant chimeras is shown. Serine addition to R69E (filled diamond) or T156K (filled triangle) mutant proteins expressed in pQE 30 had no effect on AC activity, i.e. no regulation. Mutant proteins co-expressed from a pETDUET plasmid are regulated by serine (filled squares). AC Inhibition was 35 ± 5%, and IC50 was 15 ± 5 μm (n = 4). At the right, Western blot analysis is shown of assayed proteins with the respective symbols on top (5 μg/lane for individual point mutants and 10 μg lane for jointly expressed proteins were applied). Proteins expressed concomitantly in pETDUET had N-terminal His tags, and the T156K mutant had an additional C-terminal S tag that was used for protein identification and analysis (right). For clarity the respective controls with 1 mm aspartate (in B) and 1 mm serine (in C) were omitted. The controls were negative. C, a complementation assay of Tar CyaG AC mutant chimeras is shown. Aspartate concentration-response curves for the R69E (filled diamond) or T154I (filled triangle) point mutations expressed in pQE 30 had no effect on AC activity, i.e. no regulation. Mutant proteins co-expressed from a pETDUET plasmid are regulated by aspartate (filled circles). AC Inhibition was 20 ± 4%, and IC50 was 20 ± 8 μm (n = 4). At the right, a Western blot analysis of assayed proteins with respective symbols on ths top is shown. Proteins expressed concomitantly in pETDUET had N-terminal His tags, and the T154I mutant had an additional C-terminal S tag that was used for protein identification and analysis (right). Identical amounts of protein applied to gel as in B.