FIGURE 1.
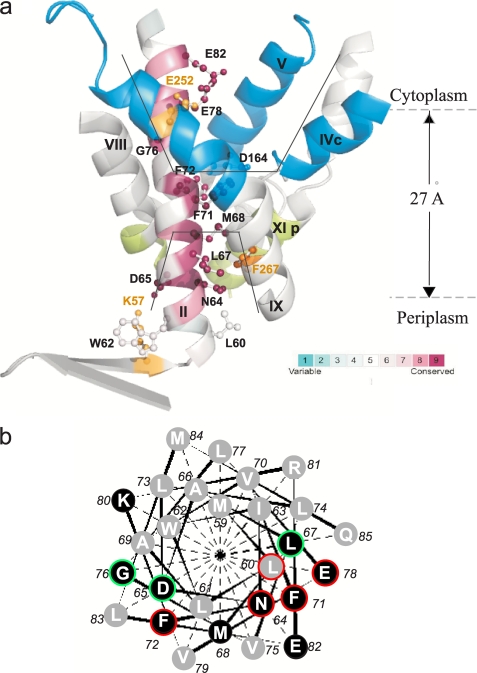
a, TMS II and the cytoplasmic and periplasmic cation funnels of NhaA. The crystal structure of helices comprising the cytoplasmic (blue) and periplasmic (green) funnels (black lines) of NhaA in addition to TMS II (maroon) are shown in ribbon representation viewed parallel to the membrane (gray broken lines). The figure was generated using the PyMOL program. TMS II is colored according to its evolutionary conservation using Consurf program. The color-coding bar, with turquoise through maroon indicates variable through conserved residues (the most variable, score 1 and the most conserved, score 9). The Cys-replaced residues on TMS II are marked in black and shown (ball and stick). For orientation, amino acids Lys57 and Glu252 are shown in yellow. b, helical wheel presentation of TMS II. A black background indicates conserved residues. Residues of which Cys replacements were alkylated by NEM are encircled in red (35–100%) or green (20–35%).