Abstract
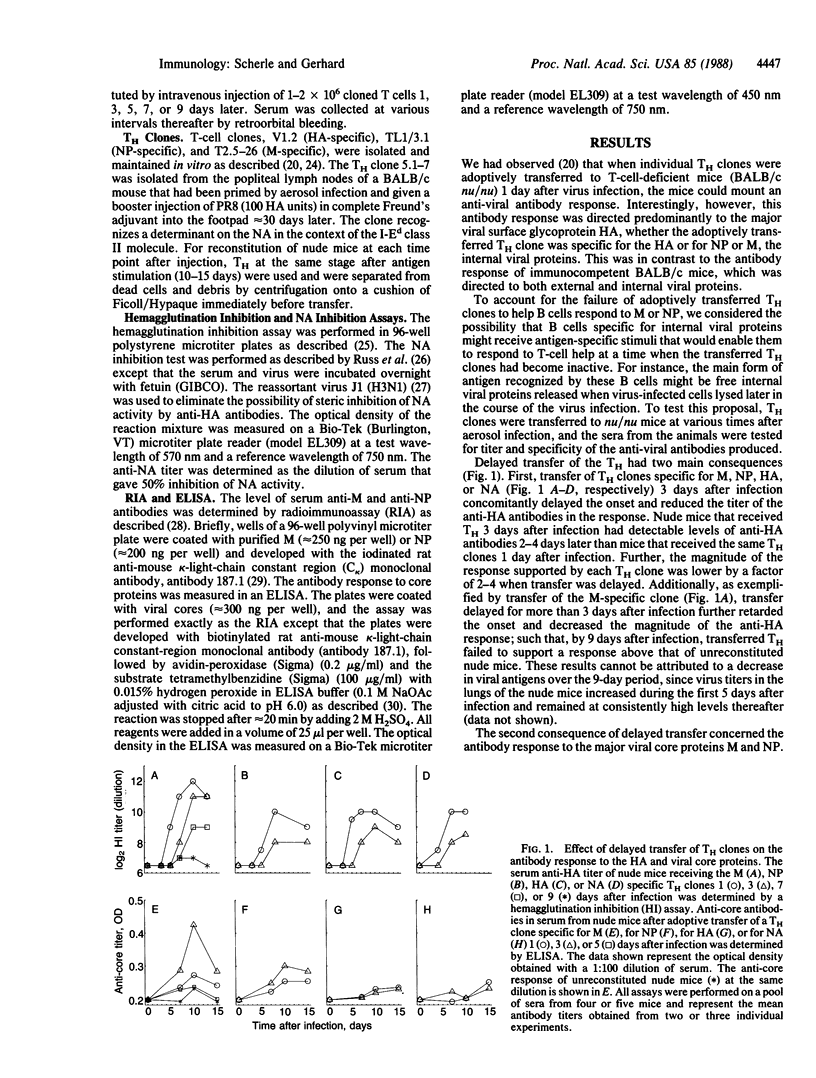
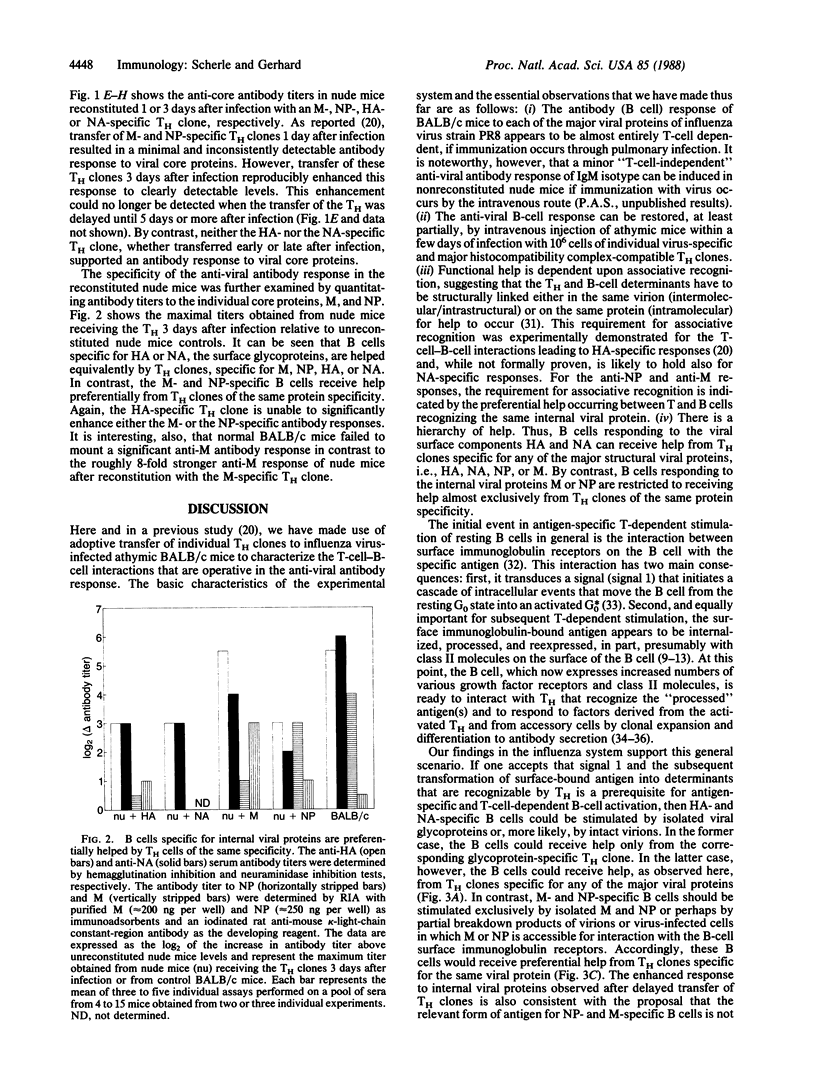
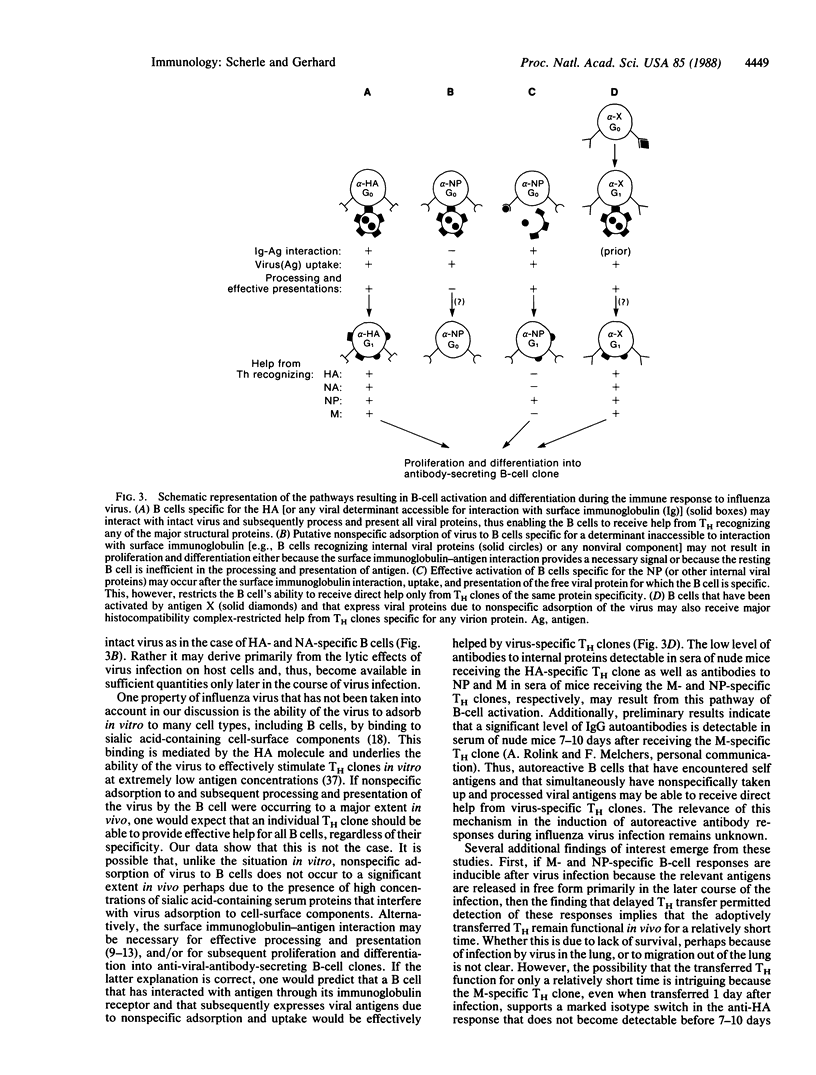
When a helper T-cell (TH) clone specific for the hemagglutinin, neuraminidase, matrix protein, or nucleoprotein of influenza strain A/PR/8/34 is adoptively transferred to athymic mice 1 day after virus infection the anti-viral antibody response of the mouse is enhanced. This response is directed predominantly to the hemagglutinin and requires associative T-cell-B-cell interactions. Delaying transfer of the TH clone has three consequences: (i) the onset of the anti-hemagglutinin antibody response is delayed; (ii) the titer of the anti-hemagglutinin response is reduced; and (iii) the titer of the antibody in the response against the internal proteins, matrix protein and nucleoprotein, is enhanced upon transfer of matrix protein- or nucleoprotein-specific, but not hemagglutinin- or neuraminidase-specific, TH clones. Thus, there is a hierarchy of help: B cells recognizing viral surface components, hemagglutinin or neuraminidase, can receive help from TH clones specific for any of the major structural viral proteins. In contrast, B cells responding to internal viral components, matrix protein or nucleoprotein, are restricted to receiving help almost exclusively from TH clones with the same protein specificity. These observations suggest that, upon B-cell surface immunoglobulin-antigen interaction and uptake of intact virus, B cells specific for viral surface proteins process and present all major structural viral antigens, enabling the B cells to interact with TH clones specific for any virion protein. B cells recognizing internal viral components, which may be accessible to interaction with B-cell immunoglobulin receptors mainly as free proteins, would present only the protein for which they are specific and, thereby, receive help only from the TH clones of the same protein specificity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Singer A., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations. Major histocompatibility complex-restricted and -unrestricted B cell responses are mediated by distinct B cell subpopulations. J Exp Med. 1981 Oct 1;154(4):1100–1115. doi: 10.1084/jem.154.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos E. S., van der Doelen A. A., van Rooy N., Schuurs A. H. 3,3',5,5' - Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoassay. 1981;2(3-4):187–204. doi: 10.1080/15321818108056977. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Dietzschold B., Wang H. H., Rupprecht C. E., Celis E., Tollis M., Ertl H., Heber-Katz E., Koprowski H. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr L. C., Gerhard W., Hackett C. J. Role of receptor-binding activity of the viral hemagglutinin molecule in the presentation of influenza virus antigens to helper T cells. J Virol. 1987 May;61(5):1375–1383. doi: 10.1128/jvi.61.5.1375-1383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Basten A. Cell interactions in the immune response in vitro. 3. Specific collaboration across a cell impermeable membrane. J Exp Med. 1972 Jul 1;136(1):49–67. doi: 10.1084/jem.136.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Hackett C., Melchers F. The recognition specificity of a murine helper T cell for hemagglutinin of influenza virus A/PR/8/34. J Immunol. 1983 May;130(5):2379–2385. [PubMed] [Google Scholar]
- Hoffeld J. T., Marrack P., Kappler J. W. Antigen-specific and nonspecific mediators of T cell/B cell cooperation. IV. Development of a model system demonstrating responsiveness of two T cell functions to HGG in vitro. J Immunol. 1976 Nov;117(5 PT2):1953–1959. [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Hackett C. J., McAndrew E. C., Gerhard W. Murine TH response to influenza virus: recognition of hemagglutinin, neuraminidase, matrix, and nucleoproteins. J Immunol. 1985 Mar;134(3):1994–1998. [PubMed] [Google Scholar]
- Johansson B. E., Moran T. M., Bona C. A., Kilbourne E. D. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. III. Reduced generation of neuraminidase-specific helper T cells in hemagglutinin-primed mice. J Immunol. 1987 Sep 15;139(6):2015–2019. [PubMed] [Google Scholar]
- Johansson B. E., Moran T. M., Bona C. A., Popple S. W., Kilbourne E. D. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. II. Sequential infection of mice simulates human experience. J Immunol. 1987 Sep 15;139(6):2010–2014. [PubMed] [Google Scholar]
- Johansson B. E., Moran T. M., Kilbourne E. D. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970 Aug 1;132(2):261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Miyake T., Nishizawa Y., Watanabe T., Yamamura Y. Triggering mechanism of B lymphocytes. I. Effect of anti-immunoglobulin and enhancing soluble factor on differentiation and proliferation of B cells. J Immunol. 1975 Nov;115(5):1179–1184. [PubMed] [Google Scholar]
- Lake P., Mitchison N. A. Regulatory mechanisms in the immune response to cell-surface antigens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):589–595. doi: 10.1101/sqb.1977.041.01.068. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Woody J. N., Hartzman R. J., Eckels D. D. In vitro influenza virus-specific antibody production in man: antigen-specific and HLA-restricted induction of helper activity mediated by cloned human T lymphocytes. J Immunol. 1982 Oct;129(4):1465–1470. [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Wortis H. H. Role of antigen-specific B cells in the induction of SRBC-specific T cell proliferation. J Immunol. 1984 May;132(5):2253–2258. [PubMed] [Google Scholar]
- Marrack P. C., Kappler J. W. Antigen-specific and nonspecific mediators of T cell/B cell cooperation. I. Evidence for their production by different T cells. J Immunol. 1975 Mar;114(3):1116–1125. [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279–304. doi: 10.1146/annurev.iy.05.040187.001431. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. B cell activation: three steps and their variations. Cell. 1984 Jul;37(3):713–720. doi: 10.1016/0092-8674(84)90407-0. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971 Jan;1(1):18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Parker D. C. Separable helper factors support B cell proliferation and maturation to Ig secretion. J Immunol. 1982 Aug;129(2):469–474. [PubMed] [Google Scholar]
- Rajewsky K., Schirrmacher V., Nase S., Jerne N. K. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969 Jun 1;129(6):1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Horan P. K. Expression of viral antigens after infection of human lymphocytes, monocytes, and macrophages with influenza virus. J Infect Dis. 1985 Feb;151(2):308–313. doi: 10.1093/infdis/151.2.308. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Liew F. Y. T cells primed by influenza virion internal components can cooperate in the antibody response to haemagglutinin. Nature. 1979 Jul 12;280(5718):147–148. doi: 10.1038/280147a0. [DOI] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E., Hünig T. Mechanism of T-cell help in the immune response to soluble protein antigens. I. Evidence for in situ generation and action of T-cell-replacing factor during the anamnestic response to dinitrophenyl keyhole limpet hemocyanin in vitro. J Exp Med. 1977 May 1;145(5):1216–1227. doi: 10.1084/jem.145.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Morrissey P. J., Hathcock K. S., Ahmed A., Scher I., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations Lyb-5+ and Lyb-5- B cell subpopulations differ in their requirement for major histocompatibility complex-restricted T cell recognition. J Exp Med. 1981 Aug 1;154(2):501–516. doi: 10.1084/jem.154.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tony H. P., Parker D. C. Major histocompatibility complex-restricted, polyclonal B cell responses resulting from helper T cell recognition of antiimmunoglobulin presented by small B lymphocytes. J Exp Med. 1985 Jan 1;161(1):223–241. doi: 10.1084/jem.161.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Bossie A., Fernandez-Botran R., Myers C. D., Oliver K. G., Sanders V. M., Stevens T. L. Interaction and activation of antigen-specific T and B cells. Immunol Rev. 1987 Oct;99:193–239. doi: 10.1111/j.1600-065x.1987.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Webster R. G., Gerhard W. U. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature. 1979 May 17;279(5710):246–248. doi: 10.1038/279246a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J., Gerhard W. Delineation of four antigenic sites on a paramyxovirus glycoprotein via which monoclonal antibodies mediate distinct antiviral activities. J Immunol. 1982 Jun;128(6):2670–2675. [PubMed] [Google Scholar]


