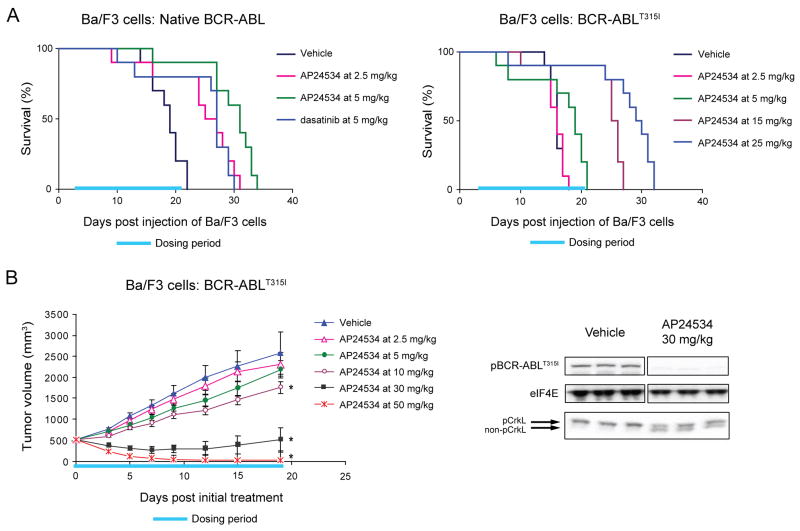
Figure 5. AP24534 is Effective in Mouse Xenograft Models of BCR-ABL-Driven and BCR-ABLT315I-Driven Tumor Growth.
(A) Effect of AP24534 on survival of SCID mice after intravenous injection of Ba/F3 cells expressing native BCR-ABL (left) or BCR-ABLT315I (right). Ba/F3 cells expressing native BCR-ABL or BCR-ABLT315I were injected into the tail vein of SCID mice, and animals were treated once daily by oral gavage with vehicle, AP24534, or dasatinib for the indicated dosing period (days 3–21).
(B) In vivo efficacy and BCR-ABL signaling suppression by AP24534 in a subcutaneous xenograft model using Ba/F3 BCR-ABLT315I cells. Tumor-bearing animals were treated once daily by oral gavage with vehicle or the indicated doses of AP24534 for 19 consecutive days (dosing period indicated) with mean tumor volume plotted (error bars represent S.E.M.). Each AP24354 treatment group was compared to the vehicle group using Dunnett’s test, with statistical significance (p<0.05) indicated by an asterisk. BCR-ABL and CrkL phosphorylation were evaluated by immunoblot in animals treated with a single oral dose of vehicle or 30 mg/kg AP24534 (N=3 per group).