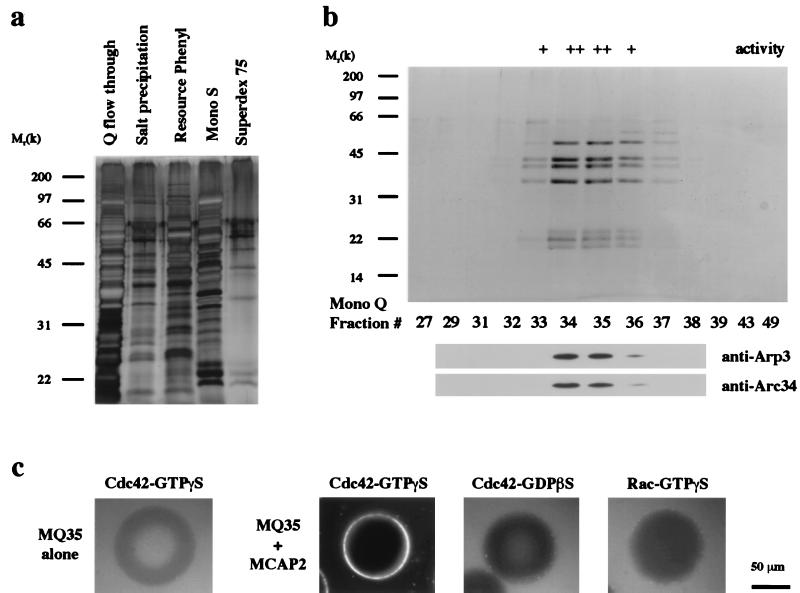
Figure 3.
Purification of MCAP1 from Xenopus egg extracts. (a) The protein compositions of the active fractions from the first five purification steps are shown on a 12.5% SDS-polyacrylamide gel stained with silver. (b) The MCAP1 activity exactly cofractionates with the Arp2/3 complex at the last purification step. Proteins from the Mono Q fractions containing and flanking the active peak were separated by 12.5% SDS/PAGE and either were stained with Gelcode Blue (Pierce) (upper) or were immunoblotted with antibodies against human Arp3 and Arc34 (lower). The activity was estimated based on the fluorescence intensity around the bead on a six-unit scale. (c) Analysis of Cdc42-induced actin polymerization in the peak fraction (MQ35, 3 μl) from the Mono Q column in the presence or absence of a MCAP2-containing fraction (3 μl) and different small GTP-binding proteins in the visual assay.