FIG. 4.
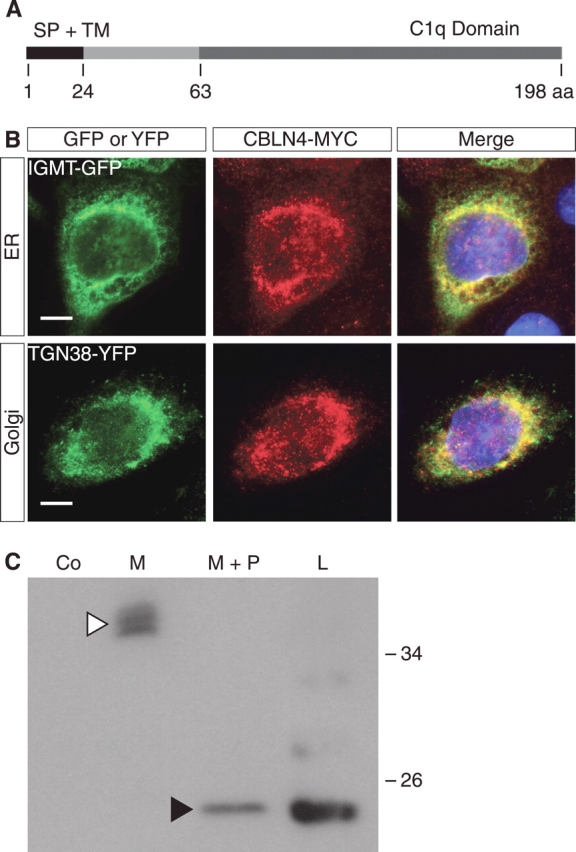
CBLN4 is a secreted protein. A) Schematic representation of CBLN4 protein with predicted signal peptide cleavage site at position 24 and C-terminal C1q domain. B) Coimmunofluorescence of MCF-7 cells transfected with the Cbln4-Myc construct and either isoprenylcysteine-O-carboxyl methyltransferase-GFP (IGMT-GFP, an endoplasmic reticulum [ER] marker; top row) or TGN38-YFP (a golgi marker; bottom row) constructs demonstrates colocalization of CBLN4-MYC with both IGMT-GFP and TGN38-YFP. DAPI staining (blue) marks cell nuclei. Bar = 10 μm. C) Secretion and deglycosylation assays performed on MCF-7 cells transfected with Cbln4-Myc construct. Western blot analysis shows that CBLN4-MYC is detected in the cell medium at approximately 38 kDa (white arrowhead). Deglycosylation results in a decrease of the molecular weight to approximately 20 kDa, similar to CBLN4-MYC from cell lysates (black arrowhead), suggesting that the difference in size of intracellular and secreted CBLN4 is the result of posttranslational glycosylation. aa, amino acid; Co, mock-transfected control; M, medium; M+P, medium with peptide-N-glycosydase; L, cell lysate; SP, signal peptide; TM, transmembrane domain.
