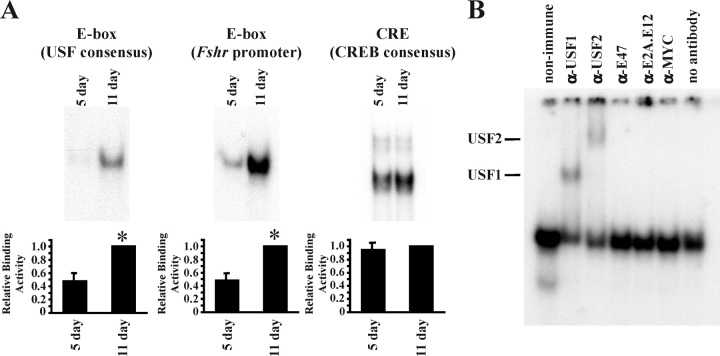
FIG. 2.
DNA-binding activity increases during Sertoli cell differentiation. Nuclear extracts isolated from Sertoli cells immediately after enzymatic digestion were assayed for DNA-protein interactions. A) In EMSAs, radiolabeled probes containing 1) a consensus E-box that is selective for USF binding, 2) a region of the Fshr promoter containing an E-box known to bind USF proteins, or 3) a CREB-binding site (CRE) were incubated with Sertoli cell nuclear extracts isolated from 5- and 11-day-old rats. Representative images of the resulting DNA-protein complexes are shown, and quantitation of the mean (±SEM) of five independent experiments is provided. The relative binding activity for each probe is normalized to that of 11-day-old Sertoli cells (=1). Statistically significant differences (P < 0.05) are indicated by an asterisk (*). B) Nuclear extracts of enriched Sertoli cells from 11-day-old rats were incubated with nonimmune sera or antisera against USF1, USF2, E47, E2A, or MYC. Supershifted DNA-protein complexes containing USF1 or USF2 are indicated. Exposure times for all complexes are less than 48 h.