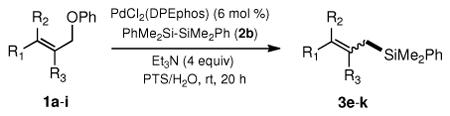
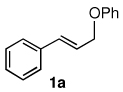
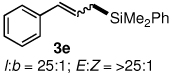
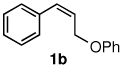
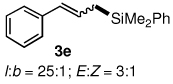
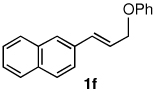
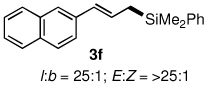
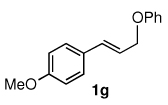
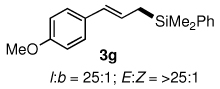
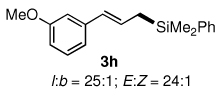
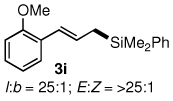
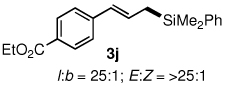
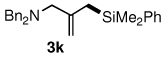
Table 3.
Pd-catalyzed silylations with 1,2-diphenyltetramethyldisilane 2b.a
All reactions were carried out on 0.25 mmol scale.
l:b and E:Z ratio determined by GCMS and 1H NMR methods.
Isolated yield after column chromatography.
10 mol % PdCl2(DPEphos) used.