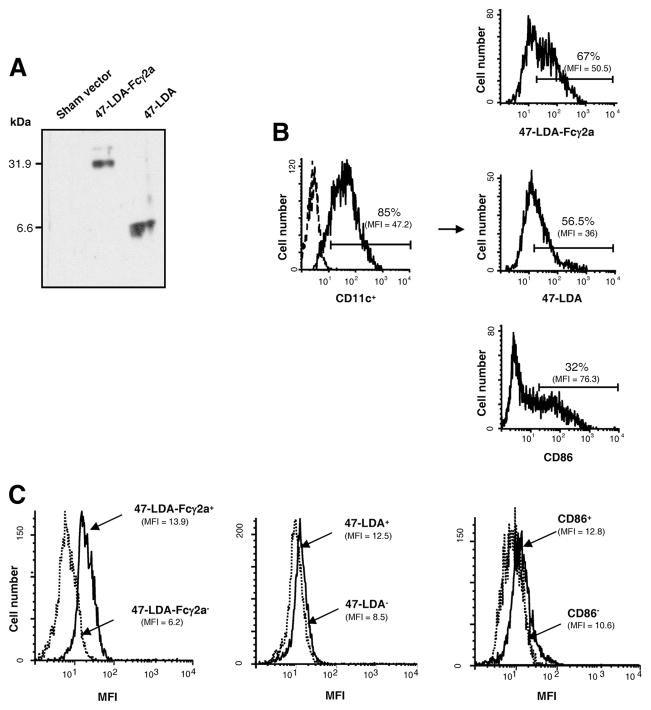
FIGURE 2.
Characterization of the 47-LDA-Fcγ2a fusion protein and its interaction with DCs. A, Western blotting of 47-LDA-Fcγ2a fusion protein and 47-LDA polypeptide with biotinylated 14G2a mAb and streptavidin-HRP. B, Binding of 47-LDA-Fcγ2a fusion protein and 47-LDA polypeptide to immature CD11c+ DCs. BM-derived DCs were stained with FITC-conjugated CD11c-specific mAb in combination with biotinylated 47-LDA-Fcγ2a fusion protein or 47-LDA polypeptide followed by streptavidin-PE and analyzed by flow cytometry. DCs stained with anti-CD86-PE mAb were included as a specificity control. The percentage and mean fluorescent intensity (MFI) of 47-LDA-Fcγ2a, 47-LDA, and CD86 molecules on CD11c-positive DCs are given. Arrows indicate the source of cells in the histograms. Data are from one representative experiment of three performed. C, Intracellular expression of IL-12p70 × 47-LDA-Fcγ2a+, 47-LDA+, CD86+ DCs, and their negative counterparts after LPS stimulation. Immature DCs were stained with biotinylated 47-LDA-Fcγ2a fusion protein, 47-LDA polypeptide, or CD86-specific mAb followed by streptavidin-PE. The positive and negative populations were sorted on a BD FACSAria flow cytometer, incubated overnight with 1 μg/ml LPS, and analyzed for IL-12p70 expression by intracellular staining with a rat anti-mouse IL-12p70 mAb specific for the IL-12p35 subunit, followed by goat anti-rat secondary Ab. Background staining (MFI, <2.6) was assessed using an isotype control Ab. All flow cytometric evaluations were performed on a FACScan or FACSCalibur flow cytometer. After gating on forward and side scatter parameters, at least 10,000-gated events were routinely acquired and analyzed using CellQuest software. Data are from one representative experiment of three performed.