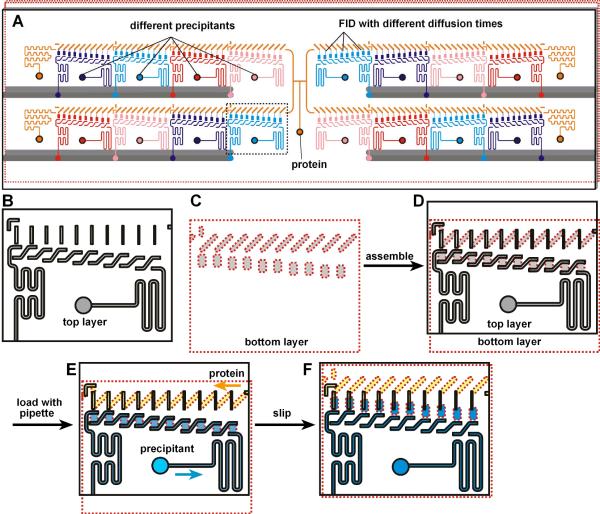
Figure 1.
A SlipChip designed to screen a protein against 16 different precipitants using the FID method of crystallization. A) A schematic of the SlipChip. Multiple precipitants (purple, blue, red, and pink), as well as multiple equilibration times for mixing the protein (orange) with each precipitant, can be screened on the same SlipChip. B–F) A zoomed-in schematic of the area outlined in A showing the operation of the SlipChp. B) The top plate (contoured in black) contains ducts for the protein and ducts for the precipitant. The ducts for the protein will become the neck channels that connect the protein wells and the precipitant wells, and these ducts gradually decrease in width from left to right, gradually changing the equilibration time. C) The bottom plate (contoured in red) has wells for the protein and wells for the precipitant. The distance between the wells for the protein and wells for the precipitant is gradually increased from left to right, gradually changing the equilibration time. D) When the two plates are assembled, the fluidic path for the protein and the fluidic path for the precipitants are formed. E) Solutions of protein (yellow) and precipitant (blue) after loading by pipetting. F) After “slipping”, protein and precipitant wells from the bottom plate are bridged by narrow channels in the top plate.