Figure 2.
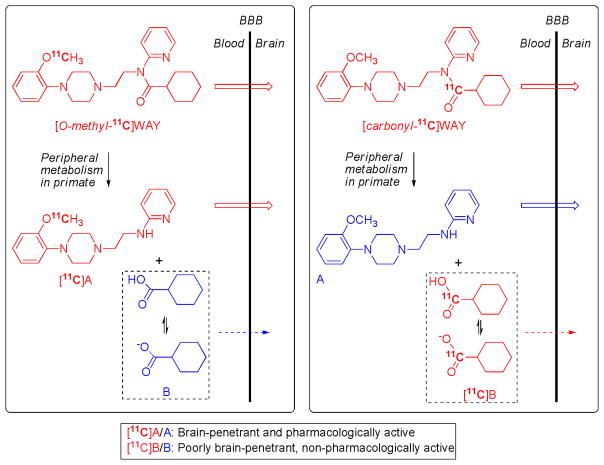
[11C]WAY-110635 for imaging brain 5-HT1A receptors: avoiding troublesome radiometabolites by judicious choice of position of radiolabel. WAY-100635 is metabolized in the periphery by hydrolysis of its amide bond. When WAY-100635 is labeled with carbon-11 in its O-methyl group, metabolism produces [11C]A as a major radiometabolite in plasma. [11C]A is able to enter brain to bind non-specifically, and also potentially specifically to some proteins for which it has moderately high affinity (5-HT1A receptors and α1 adrenoceptors) [54]. This radiometabolite therefore attenuates the signal from the unchanged radiotracer, [O-methyl-11C]WAY-100635, and exacerbates biomathematical analysis. By placing the radiolabel in the carbonyl position, metabolism of the radiotracer, [carbonyl-11C]WAY-100635, to [11C]A is avoided. The major radiometabolite becomes [11C]cyclohexanecarboxylate anion, which has only low transient entry into brain [55]. Consequently, with this radiotracer a much greater proportion of the radioactivity in brain is specifically bound unchanged radioligand [27], and biomathematical modeling of the acquired PET data is possible to deliver measures of receptor density [79, 80].