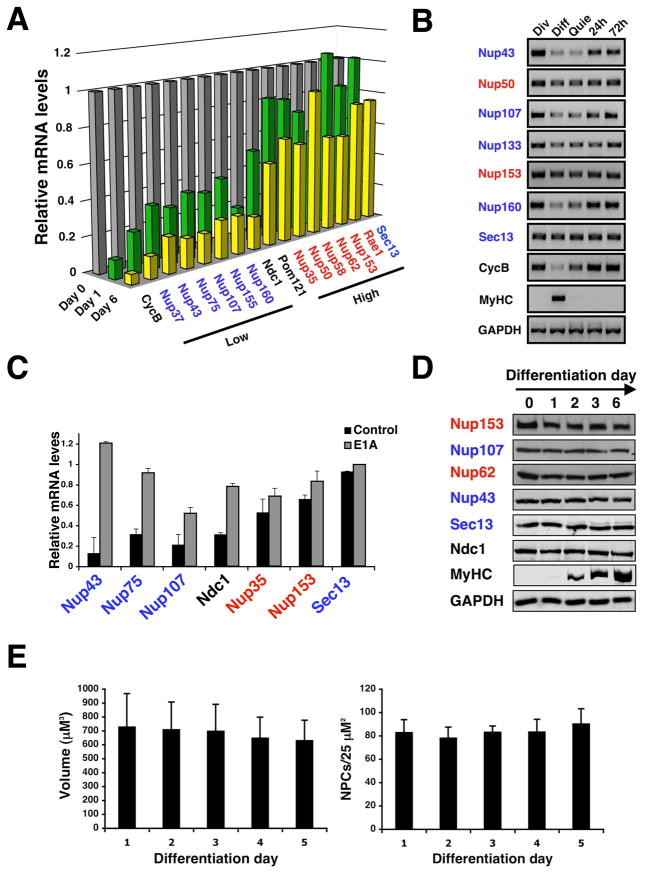
Figure 4. Down-regulation of scaffold nuceloporin expression is conserved in mammals.
(A). Total RNA was extracted from dividing C2C12 myoblasts (day 0) or differentiated myotubes (day 3 and 6). Nucleoporin expression levels were analyzed by Q-PCR (n=3). Cyclin B was used as a marker for cell cycle exit. In all cases standard deviation is below 20%. (B) Total RNA was extracted from dividing (Div), differentiated (Diff) and quiescent (Quie) C2C12 cells. Nucleoporin expression levels were analyzed by RT-PCR. Cyclin B (CycB) and myosin heavy chain (MyHC) were used and controls for cell cycle exit and differentiation respectively. (C) Dividing C2C12 myoblasts were infected with retrovirus carrying a control vector or a vector expressing the E1A protein fused to the estrogen receptor. Cells were induced to differentiate for 3–4 days and E1A was activated with 4OH-tamoxifen for 24 hours. Total RNA was extracted and nucleoporin levels were analyzed by RT-PCR (n=3). (D) Total protein was extracted from dividing myoblasts (day 0) or differentiated myotubes (day 1–6). Nucleoporin protein levels during C2C12 differentiation were analyzed by western blotting. Myosin heavy chain (MyHC) was used as a differentiation control. (E) C2C12 cells expressing 3GFP-NLS were induced to differentiate. Dividing myoblasts (Day 0) and differentiated myotubes (Day 1–5) were fixed and NPCs were stained using anti-Nup153 antibody. Nuclear volume and NPC density were analyzed by confocal microscopy and quantified.