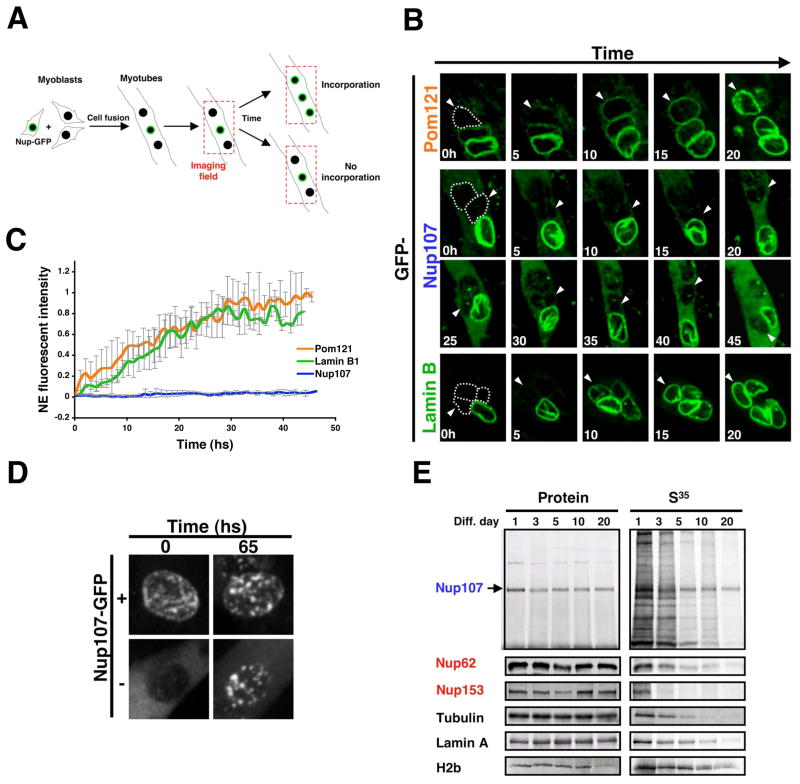
Figure 5. Nup107 scaffold nucleoporin does not exchange once inserted into NPCs.
(A). Scheme of the imaging setup used to analyze the incorporation of GFP-nucleoporins or Lamin B1-GFP fusion proteins into the NE of differentiated myotubes. (B) Proliferating myoblasts were transfected with Pom121-GFP, GFP-Nup107 or Lamin B1-GFP expressing vectors, diluted with untransfected cells and induced to differentiate for 3 days in low serum containing media. Fields containing GFP positive and negative nuclei were selected and imaged using a spinning disk confocal microscope for at least 50 hs at 1 h intervals. Dotted lines show the position of GFP negative nuclei at time 0 and arrowheads are used to follow the GFP negative nuclei during time. (C) Incorporation of Pom121-GFP, GFP-Nup107 or Lamin B1-GFP at the NE of GFP negative nuclei was quantified using Image J. (D) Proliferating myoblasts were transfected with a GFP-Nup107 expressing vector, diluted with untransfected cells and induced to differentiate for 3 days. Fields containing nuclei that came from transfected cells (+) or untransfected cells (−) were selected and imaged using a spinning disk confocal. Images show the formation of Nup107 aggregates after 65 hs of overexpression. (E) Dividing C2C12 myoblasts were incubated with a S35-Methonine/S35-Cysteine mix for 24 hs (Pulse) and then switched to differentiating media (Chase). Total cell lysates were prepared from the indicated time points. Proteins were immunoprecipitated using specific antibodies, separated by SDS-PAGE and transfered to nitrocellulose membranes. The presence of S35-labeled proteins was analyzed using a phosphoimager and protein levels were determined by western blot.