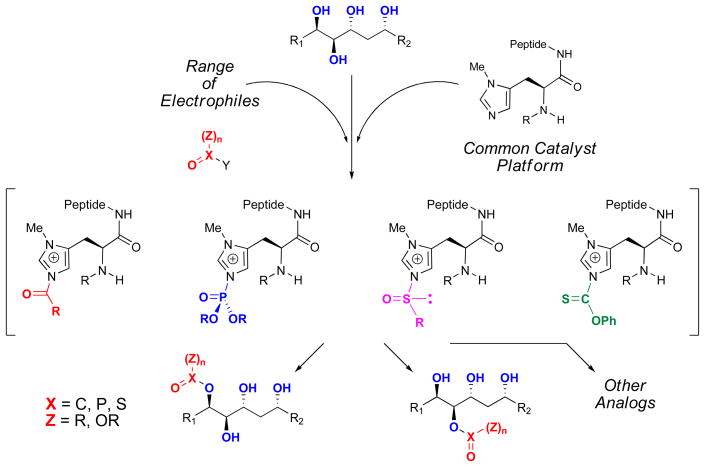
Figure 1. π-Methyl-histidine-containing peptides for group transfer chemistry.
Previous work from the Miller group has resulted in selective transfer of acyl, phosphoryl, sulfinyl, and thiocarbonyl groups to alcohols using π-methyl-histidine-containing peptides. These reactions are thought to proceed through a N-methyl imidazolium intermediate generated by nucleophilic attack of the imidazole moiety on the electrophile. Careful design and optimization of the peptide catalysts can allow for the facile synthesis of a variety of analogs of complex polyols.