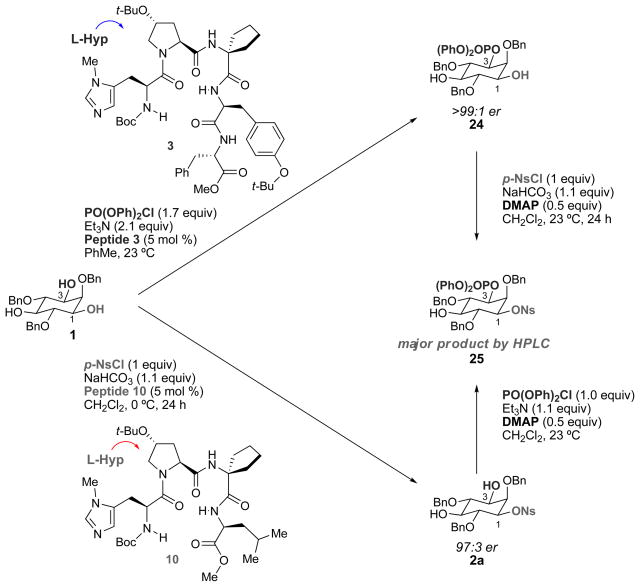
Figure 5. Determination of absolute configuration of sulfonates by comparison to phosphorylation.
(Mono(nosylated), mono(phosphorylated) inositol 25 was prepared through two independent methods, and the products were compared by HPLC retention times. From previous work, it was known that peptide 3 selectively phosphorylates inositol 1 at the 3-position to give diol 24. Sulfonylation of 24 using an achiral catalyst produces compound 25. Surprisingly, phosphorylation of mono(sulfonate) 2a obtained through nosylation mediated by peptide 10 also yielded 25. Therefore, despite the similarity between peptides 3 and 10, they act at enantiotopic alcohols for phosphorylation and sulfonylation.