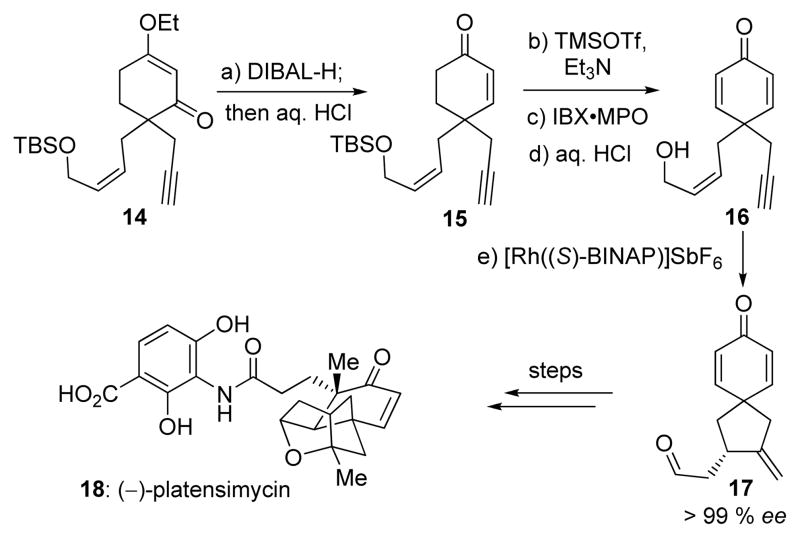
Scheme 3.
Formal total synthesis of (−)-platensimycin. Reagents and conditions: a) DIBAL-H (1.0 M in hexanes, 1.2 equiv), THF, −78 → −20 °C, 1 h; then 2 N aq. HCl, 0 °C, 30 min, 88 %; b) TMSOTf (1.2 equiv), Et3N (1.5 equiv), CH2Cl2, 0 °C, 30 min; c) IBX (1.2 equiv), MPO (1.2 equiv), DMSO, 23 °C, 3 h; d) 1 N aq. HCl, THF, 0 °C, 1 h, 68 % over three steps; e) [Rh((S)-BINAP)]SbF6 (0.05 equiv), DCE, 23 °C, 12 h, 86 %, > 99 % ee. DIBAL-H = diisobutylaluminum hydride, DMSO = dimethyl sulfoxide, IBX = o-iodoxybenzoic acid, MPO = 4-methoxypyridine-N-oxide.