Table 1.
Catalyst screening.a
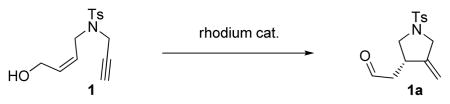 | |||
|---|---|---|---|
| Catalyst | Yield of 1a [%] | ee [%]b | |
| 1 | [Rh(cod)(MeCN)2]BF4,(S)-BINAP | 36 | 90 |
| 2 | [Rh(cod)Cl]2, (S)-BINAP, AgOTf | 60 | 91 |
| 3 | [Rh(cod)Cl]2, (S)-BINAP, AgSbF6 | 65 | 95 |
| 4 | [Rh((S)-BINAP)]SbF6 | 86 | >99 |
Reactions were run in DCE (0.4 M) in the presence of 5–10 mol % catalyst at 23°C for 12–16 h.
Measured by chiral HPLC (OD-H column) after derivatization to the corresponding p-bromobenzoate ester. BINAP = 2,2′-bis(diphenylphosphino)-1,1′-binaphthalene, cod = 1,5-cyclooctadiene, DCE = 1,2-dichloroethane.
