Table 2.
Asymmetric cycloisomerization reactions.a
| Entry | Substrate | Product | Yield[%] | ee [%] |
|---|---|---|---|---|
| 1 |
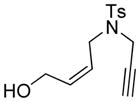 1 |
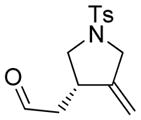 1a |
86 | >99b |
| 2 |
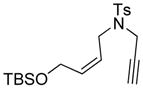 2 |
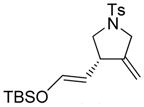 2a |
90 | 97b |
| 3 |
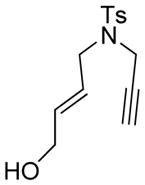 3 |
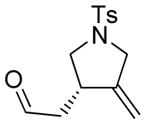 3a |
45 | 29c |
| 4 |
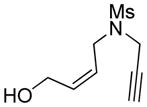 4 |
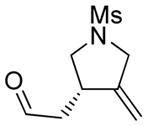 4a |
85 | >98c |
| 5 |
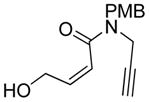 5 |
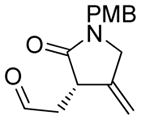 5a |
75 | 98c |
| 6d |
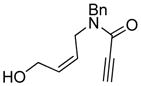 6 |
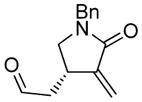 6a |
89 | 93b |
| 7 |
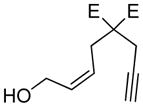 7:E=CooMe |
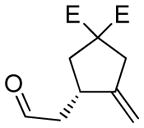 7a |
78 | 97c |
| 8d | 8:E=Cooipr | 8a | 84 | >98c |
| 9d | 9:E=So2ph | 9a | 92 | 87c |
| 10 |
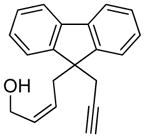 10 |
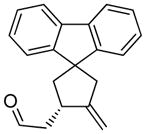 10a |
85 | >98c |
| 11 |
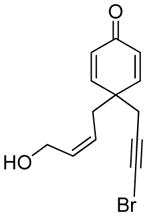 11 |
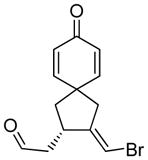 11a |
92 | >99b |
| 12d |
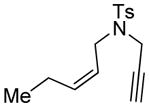 12 |
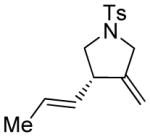 12a |
93 | >98e |
| 13d |
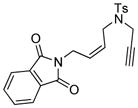 13 |
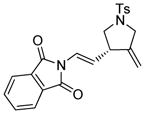 13a |
83 | 94f |
Reactions were run in DCE (0.4 M) in the presence of 10 mol % [Rh((S)-BINAP)]SbF6 at 23 °C for 12–16 h.
Measured by chiral HPLC (OD-H column) after derivatization to the corresponding p-bromobenzoate ester or ethylene glycol acetal.
Measured by 1H and 19F NMR spectroscopic analysis of the corresponding Mosher ester.
Reactions were run in acetone as solvent.
Measured by 1H NMR spectroscopic analysis of the corresponding Mosher ester prepared through sequential dihydroxylation-cleavage, reduction, and esterification.
Measured by chiral HPLC (OD-H column) after sequential acid hydrolysis, reduction, and derivatization to the corresponding p-bromobenzoate ester.
