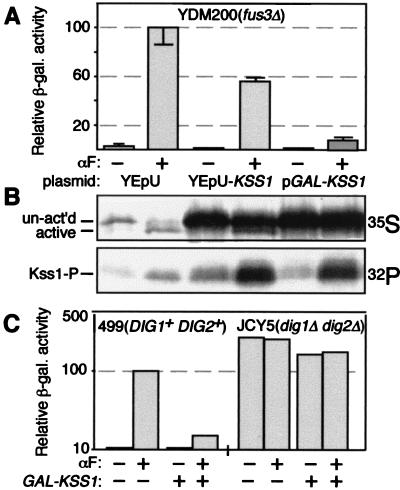
Figure 4.
Kss1 overproduction inhibits pheromone-induced transcription and Dig1 and Dig2 are required for this Kss1-imposed repression. (A) Strain YDM200 (MATa fus3Δ) was cotransformed with plasmid pJD11 containing a PRE-driven lacZ reporter and an empty vector, YEpU (bars 1 and 2), a multicopy plasmid, YEp-KSS1 ( bars 3 and 4), or a GAL-driven multicopy plasmid, YEpGAL-KSS1 (bars 5 and 6); grown to midexponential phase in medium containing 2% galactose and 0.2% sucrose; and incubated in the absence (−) and presence (+) of 6 μM α-factor mating pheromone (αF) for 2 hr. β-Galactosidase specific activity was then measured. Values are normalized to that observed for the pheromone-induced control cells (point 2; 4,500 nmol per min per mg of protein). (B) The strains described in A were labeled with either 35S (Upper) or 32P (Lower), incubated with or without 6 μM α-factor for 15 min, lysed, subjected to immunoprecipitation with polyclonal anti-Kss1 antiserum, resolved by SDS/PAGE, and analyzed by fluorography, all as described elsewhere (23). (C) Strain YPH499 (MATa DIG+) and its otherwise isogenic derivative, JCY5 (dig1Δ dig2Δ), were cotransformed with YEpU-FUS1Z and either an empty vector YCpLG (−) or YCpLG-KSS1 (GAL-KSS1) (+), grown to midexponential phase in medium containing 2% galactose and 0.2% sucrose, and incubated in the absence (−) and presence (+) of 1 μM α-factor for 90 min. β-Galactosidase specific activity was then measured. The values for vector-containing YPH499 not treated with pheromone (1.5 relative units for both points), which should lie below the abscissa, are shown as a bar just above the line to increase the clarity of presentation. Values are normalized to that observed for vector-containing YPH499 treated with pheromone (198 nmol per min per mg of protein). Standard deviations (data not shown) were less than 10% of the mean in all cases.