Table 1.
Pd-Catalyzed Coupling of Aryl Nonaflates and Triflates with Secondary Amides.a
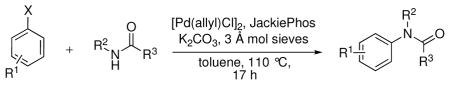 | ||||
|---|---|---|---|---|
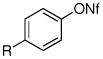
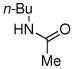
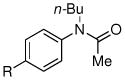
| entry | ArX | amide | product | yieldb |
|
|
|
||
| 1 | R = t-Bu | 1 R = t-Bu | 79% | |
| 2 | R = Me | 2 R = Me | 85% | |
| 3 | R = CO2Me | 3 R = CO2Me | 87% | |
| 4 |
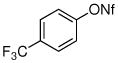
|
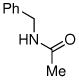
|
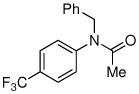
|
79% |
| 5 |

|
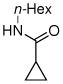
|
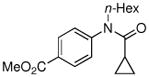
|
85% |
| 6c |

|
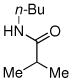
|
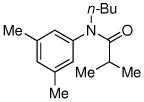
|
82% |
| 7 |
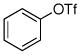
|
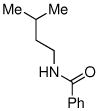
|
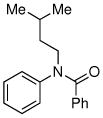
|
76% |
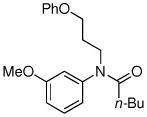
| 8 |
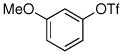
|
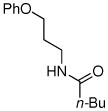
|
|
77% |
| 9 |
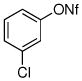
|
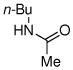
|
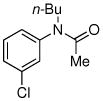
|
72% |
| 10 |
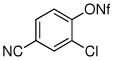
|
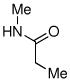
|
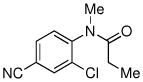
|
70% |
| 11d |
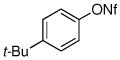
|
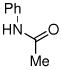
|
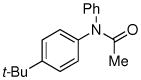
|
78% |
Reaction conditions: ArONf/ArOTf (1 equiv), amide (2.5 equiv), [(allyl)PdCl]2 (1 mol%), JackiePhos (5 mol%), K2CO3 (2.0 equiv), 3 Å mol sieves (200 mg/mmol), toluene (0.25 M), 110 °C, 17 h.
Yields reported are an average of at least two runs determined to be >95% pure by elemental analysis or 1H NMR.
Pd(OAc)2 (3 mol%), JackiePhos (7 mol%), H2O activation
K3PO4 was used instead of K2CO3.
