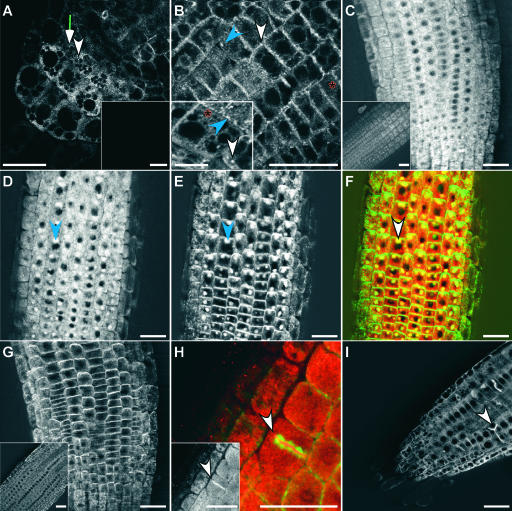
Figure 4.
Localization of GFP-ARG1 and cMyc-ARG1.
(A) and (B) Multiphoton images of transgenic arg1-2 root cap (A) and distal EZ (B) carrying the GFP-ARG1 construct, showing a large abundance of small dotted signals located at the periphery of all cells, larger punctated signals that appear circular (white arrowheads) or linear (blue arrowheads) in shape, and reticulated signals (red asterisks). Insets show signals from a nontransgenic root cap (A) and a cortical EZ cell from a different root that shows the reticulate and large punctate fluorescence patterns (B).
(C) and (D) Confocal images of the distal EZ of transgenic arg1-2 carrying a cMyc-ARG1 construct immunostained with anti-cMyc antibodies. The inset in (C) shows signals from a nontransgenic root. The seedling in (D) was treated for 2 h with 50 μM BFA before fixation, showing the accumulation of the cMyc-ARG1 fusion protein in BFA compartments (blue arrowhead).
(E) The same seedling shown in (D) treated with anti-AGR1/AtPIN2 antibodies, showing the accumulation of AGR1/AtPIN2 in similar BFA compartments (blue arrowhead).
(F) A merged image of (D) and (E), showing cMyc-ARG1 in red and AGR1/AtPIN2 in green. Yellow indicates possible colocalization. Both proteins were found in the same BFA compartments (white arrowhead).
(G) Immunostaining of cMyc-ARG1 transgenic distal EZ treated with anti-AGR1/AtPIN2 without BFA treatment, showing the localization of AGR1/AtPIN2 on basal membranes. The inset shows agr1-5, an AGR1/AtPIN2 null mutant (Chen et al., 1998), treated with anti-AGR1/AtPIN2 antibodies.
(H) Immunostaining of cMyc-ARG1 transgenic EZ treated with anti-cMyc (signals in red) and anti-α-tubulin antibodies (signals in green), showing the localization of cMyc-ARG1 at the cell plate (arrowhead). The inset shows signals from anti-cMyc antibodies only (white).
(I) Confocal image of transgenic GFP-ARG1 treated for 2 h with 10 μM oryzalin followed by a 2-h wash in liquid GM medium. GFP-ARG1 is found at aberrant cell division planes (arrowhead).
Three to 15 roots were examined for each condition. Bars = 25 μm except for the inset in (B), where the bar = 10 μm.