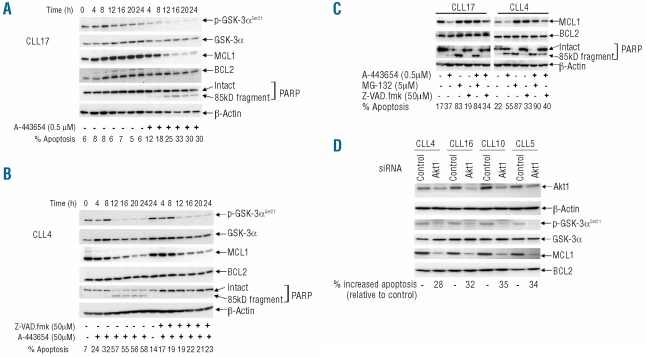
Figure 3.
Loss of MCL1 through proteasomal degradation is involved in the apoptosis induced by Akt inhibition. (A) CLL cells were cultured in the presence or absence of A-443654. Phospho-GSK-3α constituted a marker of Akt activity, while MCL1 and BCL2 were measured as relevant pro-survival proteins for CLL. Again, PARP cleavage and FACS analysis were used to examine apoptosis, while total GSK-3α and β-actin were used as loading controls. This is a representative example of five experiments on cells from five different CLL cases. (B) Cells were treated as in (A), except that the result of incubation with the pan-caspase inhibitor Z-VAD.fmk in combination with A-443654 was determined. This is a representative example of three experiments involving three different CLL clones. (C) The effect of the proteasome inhibitor, MG-132, was also analyzed. These are representative findings from four separate experiments involving four different CLL clones. In all the above experiments, the inhibitors were added to the cells 1 h prior to treatment with the Akt inhibitor. (D) The effects of knockdown of Akt1 on cell survival and levels of Mcl-1. Here, 1×107 CLL cells were mixed with 100 μL transfection solution (Amaxa) containing a total of 2 μg of siRNA duplexes or 2 μg of non-specific control siRNA before nucleofection using program X-01. Cells (5×106/mL) were subsequently cultured for 72 h, after which levels of Akt1 were measured by western blotting using an Akt1-isoform-specific antibody, while apoptosis was assessed by the FACS method. β-actin constituted a protein loading control. Reduction of Akt1 was associated with loss of p-GSK-3α and MCL1, while total GSK-3α and BCL2 were unaffected.