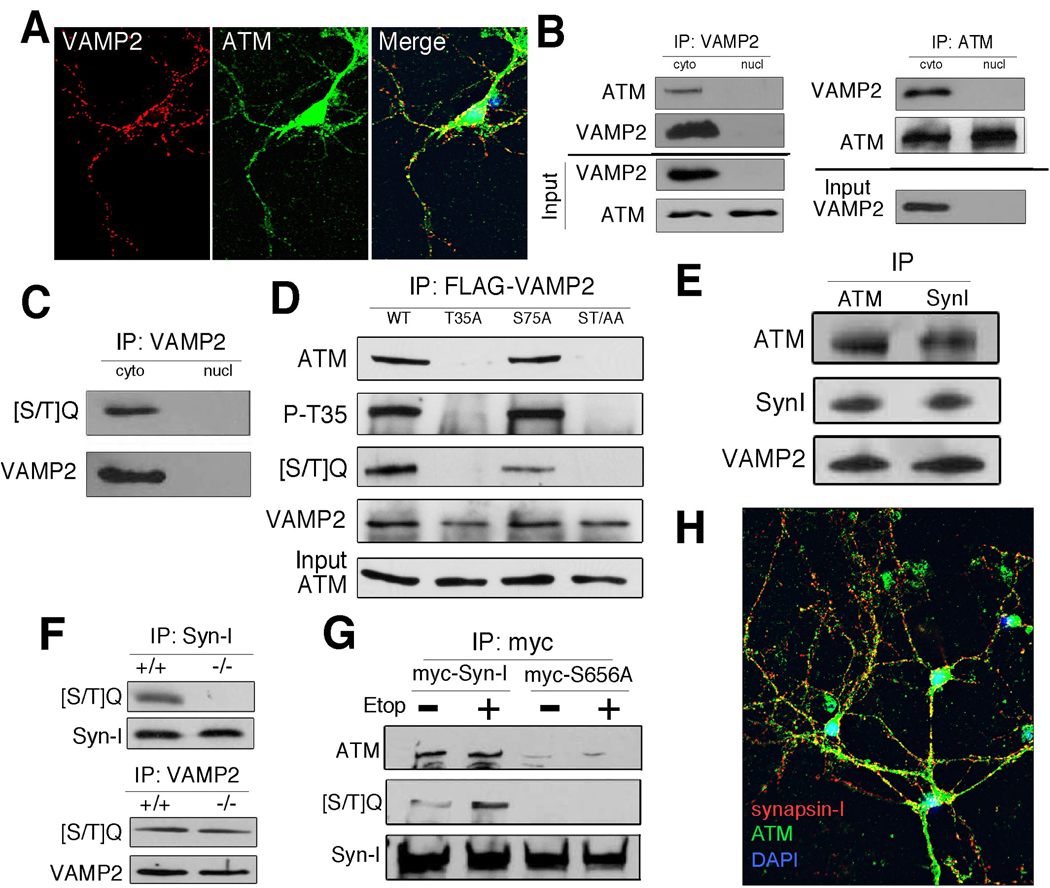
Figure 3. VAMP2 and Synapsin I are novel cytoplasmic partners of ATM in neurons.
A) VAMP2 and cytoplasmic ATM co-localization. Cultured cortical neurons were stained with antibodies against ATM (green) or VAMP2 (red). B) VAMP2 physically interacts with cytoplasmic ATM in mouse brain. Immunoprecipitations of cytoplasmic (cyto) and nuclear (nucl) extracts from 4–6 week old mouse brain were probed with the antibodies indicated. C) Neuronal VAMP2 immunoprecipitation is labeled with phospho-[S/T]Q antibody. D) VAMP2 Thr35 is phosphorylated in N2a cells, which is necessary for binding to ATM. The T35A and T35A/S75A alanine-substitution mutants lose virtually all of their phospho-T35 (P-T35) signal (2nd row), their phospho-[S/T]Q signal (3rd row) and their ability to immunoprecipitate ATM (1st row). E) Synapsin-I and ATM are physically associated. Synaptosomal protein extracts from adult mouse brain were immunoprecipitated and blotted with ATM synapsin-I or VAMP2 antibodies. F) Synapsin-I, but not VAMP2, is a enzymatic target of ATM. Cytoplasmic protein extracts from wild type (+/+) or Atmtm1Awb (−/−) mouse brain were immunoprecipitated with either synapsin-I (top) or VAMP2 (bottom) and blotted with [S/T]Q antibody. G) S656 is critical for ATM binding. Myc-tagged wild type and S656A synapsin-I were overexpressed in N2a cells, immunoprecipitated with anti-myc antibody and blotted with anti-ATM or anti-[S/T]Q. H) ATM and synapsin-I have unique but overlapping distributions in primary neuronal cultures.