Figure 4.
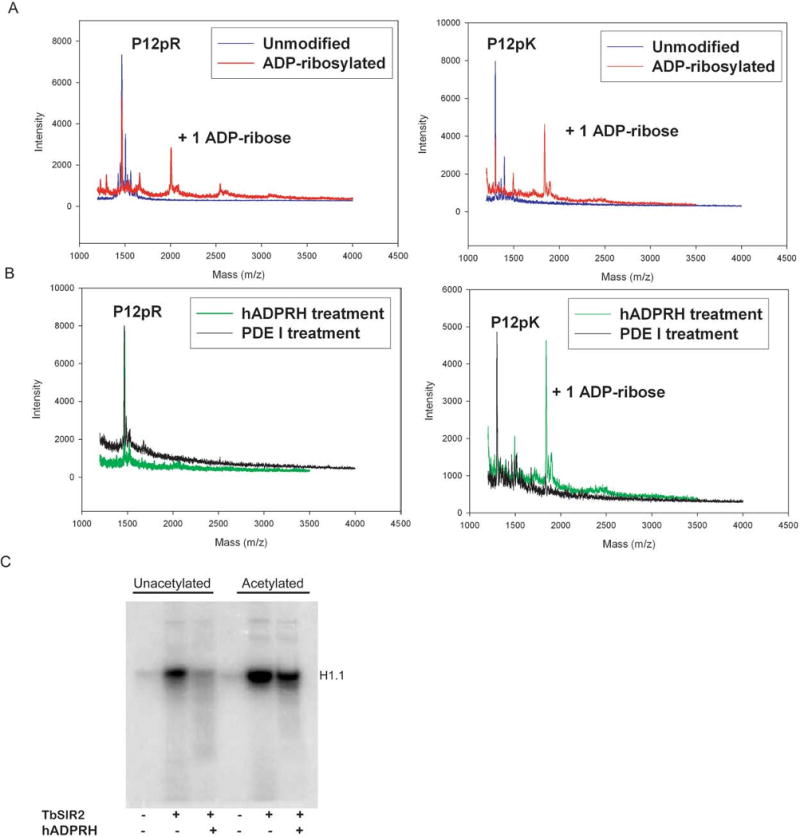
Arginine is the major ADP-ribose acceptor for unacetylated histone H1.1, but is not the major ADP-ribose acceptor for acetylated histone H1.1. (A) Nonenzymatic ADP-ribosylation of peptides P12pR and P12pK, generating an ADP-ribosylarginine substrate and an ADP-ribosyllysine substrate. (B) Enzymatic detection of ADP-ribosylarginine. Human ADP-ribosylarginine hydrolase (hADPRH) exhibits specificity for ADP-ribosylarginine, cleaving ADP-ribose from arginine side chains but not from lysine side chains. Phosphodiesterase I hydrolyzes the pyrophosphate bond for ADP-ribosylarginine and ADP-ribosyllysine. (C) Unacetylated and acetylated histone H1.1 were modified in ADP-ribosylation reactions then treated with hADPRH.
