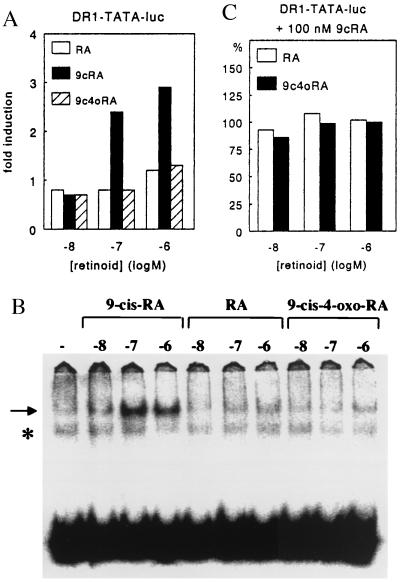
Figure 2.
(A) 9-Cis-4-oxo-RA (9c4oRA) fails to activate DR-1-TATAluc via RXRα homodimers. COS-1 cells were transfected with DR-1-TATAluc, RXRα, and SV2lacZ. (B) 9-Cis-4-oxo-RA fails to induce RXRα homodimer formation. Gel retardation assay using 32P-labeled DR-1 probe and in vitro-translated RXRα protein. Arrow indicates RXRα homodimers. ∗ indicates nonspecific complex. Retinoid concentrations indicated in log M. (C) 9-Cis-4-oxo-RA (9c4oRA) fails to compete with 9-cis-RA for activation of DR-1-TATAluc via RXRα homodimers. COS-1 cells transfected as in A). RA or 9-cis-4-oxo-RA were applied together with 10−7 M 9-cis-RA. Data are percentages relative to activation induced by 10−7 M 9-cis-RA alone. 9-Cis-4-oxo-RA has only 3-fold lower affinity for RXRα than 9-cis-RA (see Table 1), but was present in maximal 10-fold excess. Considering the lack of background caused by endogenous RXRs (not shown), we believe our experimental setting was adequate to detect any competition of 9-cis-RA by 9-cis-4-oxo-RA. We observed no competition whatsoever.