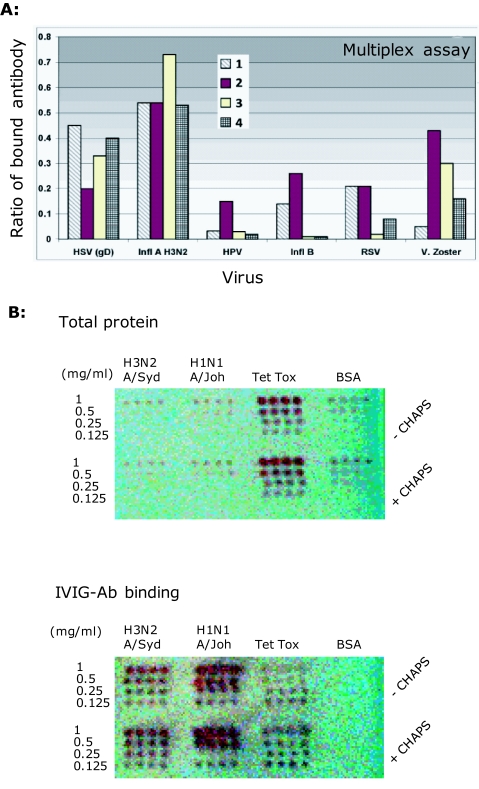
Figure 2.
Anti-human influenza binding antibodies in IVIGs: Assays for detection of Ab binding to conformational/ discontinuous influenza epitopes. A. Multiplex assay for anti-influenza IVIG Ab binding showing a comparison of the antibody binding activities of 4 different batches of a single IVIG formulation. IVIG antibody binding to Influenza A: H3N2 A/Shandong/9/1993 and Influenza B: B/Hong Kong/5/1972 antigens displayed on microbeads and performed at the Rules Based Medicine biomarker testing laboratory using multianalyte bead Fluorescence (www.rulesbasedmedicine.com). Binding to herpes simplex virus (HSV), human pappilloma virus (HPV), Influenza B (Infl B) respiratory syncytial virus (RSV) and varicella zoster (V. Zoster) are also included for comparison. The Y-axis values show the amount of IVIG antibody that binds to the respective antigens coated on beads and expressed as the mean ratio of total antibody binding to the respective viral antigens compared to highly-validated RBM internal-reference control standards. B. Microarray slides spotted with viral dilutions (1-0.125mg/ml) of H3N2 (A/Sydney/5/1997), H1N1 (A/Johannesburg/82/1996), tetanus toxoid (Tet Tox) or BSA. IVIG-antibody (D-3) bind robotically arrayed whole inactivated influenza and tetanus toxoid. The BSA is negative. Spots are 10nl and 0.8mm apart. [Note: antigens were bound in duplicate arrays with (top array panel of each slide) and without (bottom array panel on each slide) 0.005% CHAPS. CHAPS was tested as a means of improving liquid flow rates in the Piezorray, but as shown, had no influence on protein binding to the array substrate.]