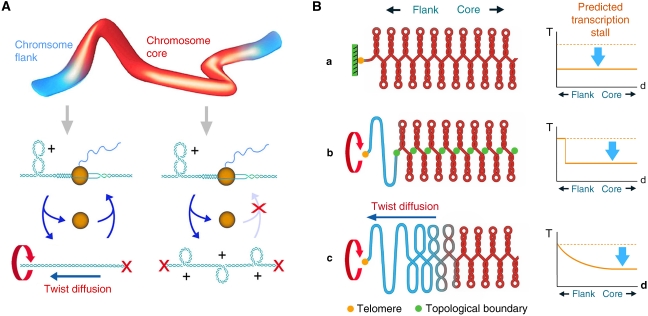
Figure 6.
Summary model. (A) Generation and diffusion of DNA helical stress. In the absence of cellular topoisomerase I and II activities and the presence of E. coli topoisomerase I, (+) helical tension is generated all along the yeast chromosomes because of unbalanced relaxation of the DNA supercoils produced during DNA transcription. In internal regions of the chromosome (core), DNA (+) helical tension cannot diffuse and accumulates until over-twisting of the duplex precludes transcription re-initiation. However, (+) helical tension can dissipate at the chromosomal ends, so allowing transcription to re-initiate at nearby regions of the chromosome (flanks). (B) DNA topological constrains along yeast chromosomes. (a) If DNA (+) helical stress could not dissipate at chromosome ends, a general stall of transcription would be expected throughout the entire chromosome. Our results discard this model. (b) If DNA (+) helical stress could dissipate at chromosome ends, but chromosomal DNA were organized as a succession of tight topological domains, a sharp transcription stall would be observed between the relaxed terminal domains and the rest of the chromosome. Our results do not support this model, unless distal topological boundaries were located beyond 100 kb from the telomere in all chromosomal arms. (c) If DNA (+) helical stress can dissipate at chromosome ends, but DNA twist diffusion is mainly restricted by the large rotational drag of chromatin, a gradual escape from the transcription stall would be expected in all chromosomal flanks, alike the observed in our results. As less DNA torque is needed in the chromosomal flanks to overcome the rotational drag of chromatin, the probability of transcription initiation is gradually restored towards the chromosomal ends.