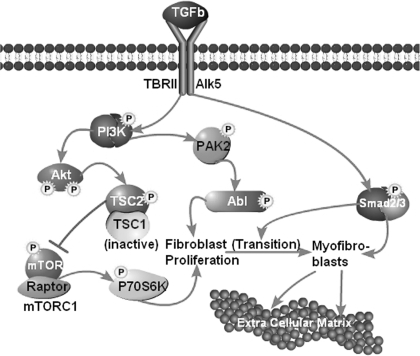
Fig. 6.
Working model of TGF-β actions in renal interstitial fibrogenesis. The canonical pathway through smad2/3 contributes primarily to the transition of fibroblasts into myofibroblasts and to the transcriptional activation of various extracellular matrix proteins in myofibroblasts as well as in epithelial and nonepithelial glomerular and tubular cells. The noncanonical (smad-independent) pathways are initiated by activation of PI3K which is a branch point for the activation of PAK2-Abl. The latter induces fibroblast proliferation, thereby increasing the number of myofibroblast precursors. PI3K also activates Akt. One of its downstream targets is TSC2 which becomes phosphorylated and thereby inactivated. Active (nonphosphorylated) TSC2 functions as a GTPase-activating protein (GAP) protein converting mTORC1-activating Rheb-GTP to Rheb-GDP. Thus, active TSC2 is an inhibitor of mTORC1, and loss of TSC2 activity by phosphorylation increases mTORC1 activity. This activates downstream substrates including P70S6 kinase, a translational activator of many proteins including cell cycle proteins and hence, proliferation. The Akt → mTORC1 → P70S6K branch pathway contributes also to fibroblast proliferation. Thus, in fibroblasts, the noncanonical and canonical TGF-β pathways closely collaborate: the noncanonical pathway induces fibroblast proliferation and the canonical smad2/3 pathway induces transition of the increased number of fibroblasts into active myofibroblasts and acts in these latter cells as transcriptional activator of multiple extracellular matrix proteins as well as regulators of matrix accumulation.