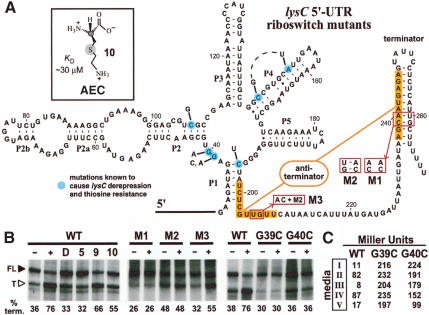
Figure 5.
The B. subtilis lysC riboswitch and its mechanism for metabolite-induced transcription termination. (A) Sequence and repressed-state model for the lysC riboswitch secondary structure. The nucleotides highlighted in orange identify the putative antiterminator interaction that could form in the absence of L-lysine. Boxed nucleotides identify sites of disruption (M1) and compensatory mutations for the terminator stem (M2) and for the terminator and antiterminator stems (M3). Nucleotides shaded in light blue identify some of the positions in which mutations exhibit lysC derepression that were reported previously (Vold et al. 1975; Lu et al. 1992). These mutations confer resistance to AEC (inset). (B) Single-round in vitro transcription termination assays conducted in the absence (-) or presence (+) of 10 mM L-lysine or other analogs as indicated. (FL) Full-length transcripts; (T) terminated transcripts. The percent of the terminated RNAs relative to the total terminated and full-length transcripts are provided for each lane (% term.). (C) In vivo expression of a β-galactosidase reporter gene fused to wild-type (WT), G39A, and G40A mutant lysC 5′-UTR fragments. Media conditions are as follows: (I) normal medium (0.27 mM lysine); (II) minimal medium (0.012 mM); (III) lysine-supplemented minimal medium (1 mM); (IV) lysine hydroxamate-supplemented (medium II plus 1 mM lysine hydroxamate) minimal media; (V) AEC-supplemented (medium II plus 1 mM AEC) minimal medium.