Abstract
Hypoxia, or reduced oxygen, occurs in a variety of clinical and environmental situations. Hypoxic exposure is associated with decreased muscle mass and a concomitant reduction in exercise capacity, although the exact mechanisms are not completely understood. The activin type IIB receptor (ActRIIB) is a receptor for transforming growth factor-β (TGFβ) superfamily members that are involved in the negative regulation of lean tissue mass. Given that hypoxia has negative effects on muscle mass and function and that modulation of the ActRIIB has been shown to increase muscle mass, we tested the hypothesis that pharmacological targeting of the ActRIIB for 2 wk would attenuate the loss of muscle mass and function in mice after exposure to normobaric hypoxia. ActRIIB modulation was achieved using a soluble activin receptor/Fc fusion protein (sActRIIB) in mice housed in a hypoxic chamber for 1 or 2 wk. Hypoxia induced a reduction in body weight in PBS- and sActRIIB-treated mice, although sActRIIB-treated mice remained larger throughout the hypoxic exposure. The absolute forces generated by extensor digitorum longus muscles were also significantly greater in sActRIIB- than PBS-treated mice and were more resistant to eccentric contraction-induced force drop after eccentric lengthening contractions. In summary, sActRIIB pretreatment attenuated hypoxia-induced muscle dysfunction. These data suggest that targeting the ActRIIB is an effective strategy to counter hypoxia-induced muscle dysfunction and to preacclimatize to hypoxia in clinical or high-altitude settings.
Keywords: activin receptor, myostatin, muscle wasting, muscle physiology
exposure to a severely hypoxic environment leads to a loss of body and muscle mass (10, 13, 19). The loss of muscle mass accounts for a large percentage of the overall reduction in body mass (10, 19). Accompanying this loss of muscle mass is a reduction in the force-generating capacity of skeletal muscle, indicative of a hypoxia-induced muscle dysfunction. Muscle dysfunction resulting from hypoxic exposure can significantly alter exercise performance (7, 12), as well as activities of daily living in disease situations (11). Indeed, exposure of the mdx mouse model of Duchenne muscular dystrophy (DMD) to intermittent periods of hypoxia has been shown to further aggravate the contractile dysfunction of the diaphragm muscle (9). The exact molecular mechanisms of hypoxia-mediated muscle loss and dysfunction are not fully understood, and there are no therapies to enhance muscle growth and/or attenuate the debilitating loss of muscle mass seen with hypoxic exposure.
Control of muscle mass is a complex process, and regulation is achieved, in part, by a complex interplay of genetic predisposition, developmental programming, presence or absence of injury and/or disease, environmental influences, and growth or developmental factors. The activin type IIB receptor (ActRIIB) is a transmembrane serine-threonine kinase receptor for multiple transforming growth factor-β (TGFβ) superfamily members that are involved in the negative regulation of lean muscle tissue, including activin A, nodal, bone morphogenic protein (BMP)-2, BMP-6/7, growth and differentiation factor (GDF)-5, and GDF-8/11 (myostatin) (8). Inhibition of ActRIIB signaling by a soluble decoy receptor results in dramatic increases in skeletal muscle mass (5, 17). Humans and a number of animal species, including cattle, dogs, mice, and sheep, with genetic mutations in the myostatin gene also exhibit increased muscularity (15, 16, 21, 22, 24, 25). Myostatin-blockade strategies have also been used experimentally to reduce myostatin activity (27). Pharmacological inhibition of myostatin has been shown to increase muscle mass and improve functional characteristics of dystrophic skeletal muscle in the mdx mouse (2, 3, 26).
In the present study, we tested the hypothesis that targeting the ActRIIB pharmacologically would attenuate muscle mass loss, as well as improve the muscle dysfunction that occurs after exposure to hypoxia. Wild-type mice were treated for 2 wk with a novel, soluble ActRIIB molecule (sActRIIB) and then exposed to hypoxia for 1 and 2 wk. We used a combination of physiological and histological methods to analyze sActRIIB-treated and PBS control cohorts of mice and quantify the attenuation of hypoxia-mediated muscle dysfunction.
MATERIALS AND METHODS
Mice.
Male C57BL/10ScSn mice were purchased from Jackson Laboratories at 8 wk of age and acclimated to the animal facility at the University of Pennsylvania for 2 wk before initiation of the experiments. A maximum of four mice were housed together in individual cages during this acclimatization period. Mice were exposed to a 12:12-h light-dark cycle and 22°C ambient temperature and received rodent chow (Lab Diet 5001) and water ad libitum. Mice were randomly assigned to a PBS-treated control group exposed to 1 wk of hypoxia (n = 8 mice) or 2 wk of hypoxia (n = 8 mice) or an sActRIIB-treated group exposed to 1 wk of hypoxia (n = 8 mice) or 2 wk of hypoxia (n = 8 mice), with experiments beginning at 10 wk of age. Control and sActRIIB-treated mice were housed in separate cages before hypoxia exposure. Age-matched C57BL/10ScSn mice (n = 6) were maintained in normoxic conditions and used as untreated controls. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
sActRIIB treatment strategy.
The ActRIIB was targeted using a fusion protein consisting of a form of the extracellular domain of ActRIIB linked to a murine Fc (sActRIIB; a kind gift from Acceleron Pharma). The methods for expression and purification of sActRIIB, as well as binding kinetics and myostatin inhibitory data, have been previously described (23). sActRIIB was used at a concentration of 10 mg/kg body wt, which was based on dose-response studies in which body mass and muscle mass showed no additional increases above this dose (14) and has been recently published in two additional mouse models (1, 23). Control mice received an equal volume of sterile PBS. Each mouse was weighed daily and injected intraperitoneally twice per week with a volume of drug based on the animal's body weight. All mice received four injections of PBS or sActRIIB during a 2-wk pretreatment period. After this pretreatment period, PBS control and sActRIIB-treated mice were placed into a hypoxic chamber for an additional 1 or 2 wk. The twice-weekly injections of PBS and sActRIIB were maintained during hypoxic exposure.
Hypoxic exposure.
The hypoxic chamber had a total gas volume of 12 liters and was maintained at 22°C and 30% humidity (Fig. 1). The hypoxic protocol is presented in Table 1. A gas mixer (Pegas 4000MF, Columbus Instruments) was used to mix the varying percentages of oxygen and nitrogen gas (Table 1), which entered the hypoxic chamber at 2.0 l/min. Mice were briefly removed from the hypoxic chamber each day so that cage bedding could be changed and mice could be weighed and/or receive injections. The hypoxic protocol was designed to simulate the hypoxic environment experienced during an ascent to an altitude of 6,500 m above sea level. The protocol was based on actual altitude ascent profiles achieved during an ascent of Mt. Meru in the Garwhal Himalayas by the Indo-Swedish expedition in 1986. After the 1- or 2-wk hypoxic exposure, mice were euthanized, and the extensor digitorum longus (EDL) muscles were prepared for ex vivo physiological measurements and then immediately flash frozen in isopentane cooled to the temperature of liquid nitrogen and stored at −80°C for muscle morphological and histological studies.
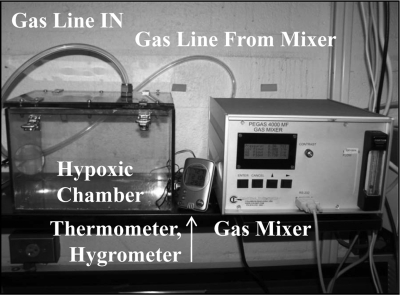
Fig. 1.
Hypoxic chamber set-up. A specially designed hypoxic chamber was used to expose mice to varying percentages of oxygen and simulate a mountain ascent to 6,500 m above sea level. Nitrogen and oxygen gas entered the gas mixer, which was used to mix the varying percentages of oxygen and nitrogen gas, and the mixtures flowed into the hypoxic chamber at 2.0 l/min. A probe was inserted into the hypoxic chamber to ensure that temperature and humidity were maintained throughout exposure to hypoxia.
Table 1.
Protocol for exposure to hypoxia
| Study Day | Po2, mmHg | O2, % | N2, % | Simulated Altitude, m |
|---|---|---|---|---|
| 15 | 118 | 15 | 85 | 1,830 |
| 16 | 100 | 13 | 87 | 3,050 |
| 17 | 91 | 12 | 88 | 3,660 |
| 18 | 83 | 11 | 90 | 4,270 |
| 19 | 76 | 10 | 90 | 4,880 |
| 20 | 69 | 9 | 91 | 5,490 |
| 21 | 63 | 8 | 92 | 6,100 |
| 22 | 76 | 10 | 90 | 4,880 |
| 23 | 69 | 9 | 91 | 5,490 |
| 24 | 69 | 9 | 91 | 5,490 |
| 25 | 69 | 9 | 91 | 5,490 |
| 26 | 63 | 8 | 92 | 6,100 |
| 27 | 63 | 8 | 92 | 6,100 |
| 28 | 63 | 8 | 92 | 6,100 |
Growth curves.
Inasmuch as muscle mass is a major contributor to body weight, growth curves offer an indirect measure of the rate of change in muscle mass (i.e., due to sActRIIB treatment and/or duration of hypoxic exposure). Mice were weighed individually every day, and weights were plotted to generate growth curves.
Ex vivo physiological assessment of skeletal muscle.
Physiological measurements, including contraction time, half-relaxation time, peak isometric twitch force, peak isometric tetanic force, and decline in eccentric contraction-induced force, were analyzed in freshly dissected EDL muscles from 12- to 13-wk-old mice, as previously described (2). Muscle length was adjusted to obtain the maximal twitch response, and this length was measured and recorded as optimal length (Lo). Peak twitch and tetanus forces were obtained from three maximal contractions, as previously described (120-Hz stimulation frequency, 500-ms stimulation duration) (2, 3). Eccentric lengthening contractions were performed as previously described (700-ms supramaximal stimulus, 500-ms isometric phase, 200-ms eccentric phase, 80-Hz stimulation frequency, total lengthening Lo/10, lengthening velocity 0.5 Lo/s) (2, 3). Force drop was calculated as the percent difference in peak force between the fifth and the first eccentric contraction of the eccentric contraction protocol. Muscles were stimulated in a Ringer solution composed of (in mM) 100 NaCl, 4.7 KCl, 3.4 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 25 HEPES, and 5.5 d-glucose. Muscle cross-sectional area (CSA) was calculated by dividing the muscle mass by the product of the muscle density coefficient (1.06 g·cm3), muscle optimal length (Lo), and fiber length coefficient for EDL muscle (0.45) (4, 18). After EDL dissection, the soleus, gastrocnemius, tibialis anterior, and quadriceps muscles were dissected, trimmed of excess connective tissue, and weighed for determination of the effects of sActRIIB and hypoxia on additional lower limb muscles.
Muscle histology.
Serial frozen sections (12 μm thick) of EDL muscle were obtained using a cryostat at −21°C and placed onto glass slides (Superfrost/Plus, Fisher Scientific). Sections were fixed in ice-cold methanol for 5 min and then processed for histological examination by hematoxylin and eosin-phloxine staining. Digital images were acquired using an Olympus BX51 microscope at an objective magnification of ×40 and a Magnafire digital camera and software. Morphometric measurements (i.e., single-fiber area and total number of fibers) were made using the Image J image-processing software (rsbweb.nih.gov/ij). To determine the single-fiber area distribution, we measured the area of 100 random muscle fibers for each muscle section. These areas were used to calculate an average single-fiber area and plot it as a histogram. We determined the total number of fibers by manually counting all the muscle fibers in hematoxylin-and-eosin-stained images.
Statistical analyses.
The present study investigates the effects of two independent variables (i.e., sActRIIB treatment and duration of hypoxia) on the physiological properties of skeletal muscle. For this reason, all data were analyzed using a two-way ANOVA to examine the main effect of hypoxic exposure (i.e., 1 wk vs. 2 wk of exposure), the main effect of sActRIIB (i.e., PBS control vs. 10 mg/kg sActRIIB), and the hypoxia × sActRIIB interaction. Values are means ± SE. Significance was set at P < 0.05.
RESULTS
Changes in body weight.
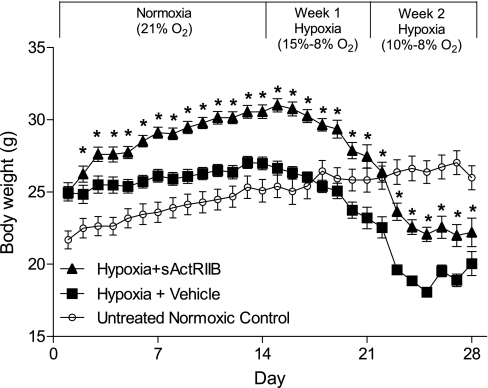
All mice were weighed daily, and the results are plotted in Fig. 2. At the start of the experiment, there were no significant differences in body weight between groups (24.9 ± 2.5 and 25.0 ± 1.7 mg for PBS- and sActRIIB-treated mice, respectively, P = 0.93). At the end of the 2-wk sActRIIB pretreatment period, sActRIIB-treated mice were significantly heavier than control mice [26.7 ± 1.9 vs. 31.0 ± 1.9 mg (+16.3%), P < 0.0001]. Calculation of the slope of the line for the PBS control and sActRIIB-treated mice reflected the increase in body mass that occurred during the 2-wk pretreatment period (slope = 0.16 and 0.43 for PBS and sActRIIB, respectively). After 1 wk of exposure to hypoxia, both cohorts of mice lost body weight; however, the sActRIIB-treated mice lost 10.1% of their body weight (27.9 g) and remained above their baseline body weight of 25.01 g (slope = −0.58). Control mice lost 13.0% of their body weight (23.2 g) and fell below their baseline body weight of 24.9 g (slope = −0.59). After 2 wk of exposure to hypoxia, sActRIIB-treated mice lost 28.4% of their body weight (slope = −0.69), whereas PBS control mice lost 24.8% of their body weight (slope = −0.42). At the completion of the trial, sActRIIB-treated mice remained 10.8% heavier than control mice (20.1 ± 2.4 vs. 22.2 ± 2.8 g, P = 0.008). As a comparison, the growth curve for the untreated normoxic control mice showed a small 4.5% increase during this 4-wk time period.
Fig. 2.
Body weight changes in response to treatment with soluble activin type IIB receptor (sActRIIB) and exposure to hypoxia. Mice were weighed daily, and growth curves were plotted during the 2-wk sActRIIB pretreatment protocol and subsequent hypoxia exposure. Body weight of mice was significantly greater in sActRIIB-treated mice than in control mice at all times after initiation of sActRIIB treatment. Hypoxia induced body weight losses, such that body weight of PBS control mice was below baseline during the 1st wk of exposure, whereas body weight of sActRIIB-treated mice remained above baseline until the 2nd wk of hypoxia. Growth curve of a separate untreated normoxic control group is plotted for comparison. *Significantly different from hypoxia + vehicle.
Changes in muscle weights.
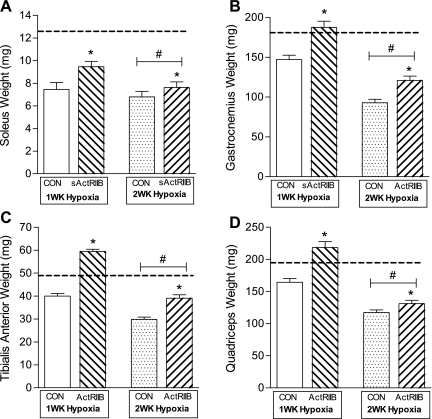
The muscles of the lower limb (soleus, gastrocnemius, tibialis anterior, and quadriceps) were dissected, trimmed of excess connective tissue, blotted dry, and weighed for determination of the effects of sActRIIB treatment and duration of hypoxia on muscle mass (Fig. 3). For each of the four muscles, a main effect of sActRIIB treatment was observed, indicating that muscles from mice treated with sActRIIB remained larger than muscles from PBS control mice after exposure to hypoxia, regardless of duration of hypoxia. In addition, a main effect of duration of hypoxia was observed for all four muscles, indicating that muscles from mice exposed to 2 wk of hypoxia were smaller than muscles from mice exposed to 1 wk of hypoxia, regardless of sActRIIB treatment. Significant statistical interactions (hypoxia × ActRIIB) were observed for the tibialis anterior (P < 0.0001; Fig. 3C) and quadriceps (P < 0.001; Fig. 3D) muscles.
Fig. 3.
Changes in skeletal muscle weight in response to sActRIIB and hypoxia. Effects of sActRIIB and hypoxia were analyzed in 4 lower limb muscles: soleus (A), gastrocnemius (B), tibialis anterior (C), and quadriceps (D). A main effect of sActRIIB treatment was observed for all muscle weights, such that muscles from sActRIIB-treated mice were larger than muscles from PBS control (Con) mice. A main effect of hypoxia exposure was observed, such that muscles were smaller after 2 wk of hypoxia than after 1 wk hypoxia. Dashed line represents muscle weights for untreated normoxic control mice. *Significant main effect of sActRIIB treatment. #Significant main effect of hypoxia duration.
Ex vivo physiological properties of the EDL muscle.
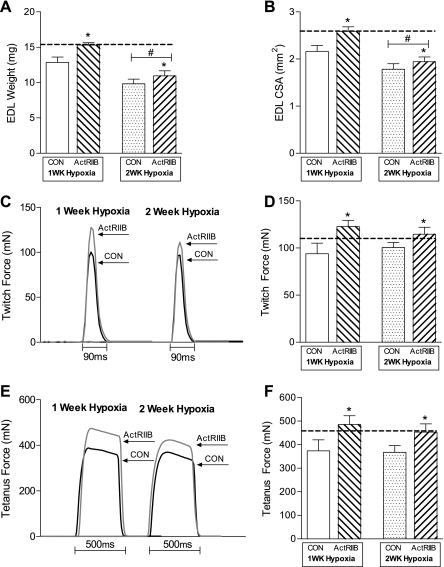
To determine whether sActRIIB treatment affected muscle function after exposure to hypoxia, we studied freshly dissected EDL muscles ex vivo. A significant main effect of sActRIIB treatment was observed for EDL muscle mass (P < 0.05; Fig. 4A) and CSA (P < 0.05; Fig. 4B), indicating that EDL muscles from sActRIIB-treated mice were larger than EDL muscles from PBS control mice. There was also a main effect of hypoxic exposure, such that the EDL muscle mass (P < 0.0001) and CSA (P < 0.0001) were smaller after 2 wk of hypoxia than after 1 wk of hypoxia.
Fig. 4.
Extensor digitorum longus (EDL) muscle and force properties in response to sActRIIB and hypoxia. A: a main effect of sActRIIB was observed in the absolute weight of the EDL muscle, such that EDL muscles from sActRIIB-treated mice were significantly larger than EDL muscles from PBS control mice. A main effect of hypoxia exposure was observed, such that EDL muscles were smaller after 2 wk of hypoxia than after 1 wk of hypoxia. B: a main effect of sActRIIB was observed in cross-sectional area (CSA) of the EDL muscle, such that CSA of EDL muscles from sActRIIB-treated mice was significantly larger than CSA of EDL muscles from PBS control mice. A main effect of hypoxia was observed, such that the CSA of EDL muscles was smaller after 2 wk of hypoxia than after 1 wk of hypoxia. C: representative twitch traces of EDL muscles. D: when absolute force during a twitch contraction of the EDL muscle was quantified, a main effect of sActRIIB treatment was observed, such that absolute force was larger in sActRIIB-treated than in PBS control mice. E: representative tetanus traces of EDL muscles. F: when absolute force during a tetanus contraction of the EDL muscle was quantified, a main effect of sActRIIB treatment was observed, such that absolute force was greater in sActRIIB-treated mice than in PBS control mice. Dashed line represents values for untreated normoxic control mice. *Significant main effect of sActRIIB treatment. #Significant main effect of hypoxia duration.
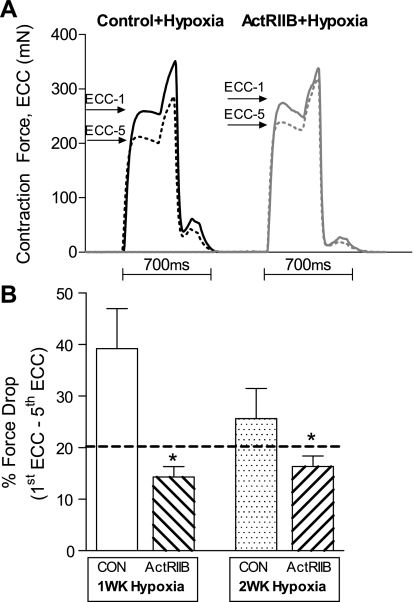
The force-generating capacity of the EDL muscle was examined ex vivo during isometric and eccentric lengthening contractions. A significant main effect of sActRIIB treatment was observed in the absolute force produced during an isometric twitch contraction, indicating that force production from EDL muscles was higher in sActRIIB-treated mice than in PBS control mice, regardless of duration of hypoxia (P < 0.05; Fig. 4, C and D). Similarly, a significant main effect of sActRIIB treatment was observed in the absolute force produced during an isometric tetanic contraction, indicating that force production in EDL muscles was higher in sActRIIB-treated mice than in PBS control mice, regardless of duration of hypoxia (P < 0.05; Fig. 4, E and F). As evidence of functional improvement of skeletal muscle exposed to hypoxia, a main effect of sActRIIB treatment was observed in the force drop following five eccentric lengthening contractions (P < 0.05; Fig. 5). These data indicate that EDL muscles from sActRIIB-treated mice were more resistant to eccentric lengthening contraction-induced injury than muscles from PBS control mice, regardless of duration of hypoxia.
Fig. 5.
Eccentric lengthening contraction induced force drop in response to sActRIIB treatment and hypoxia exposure. A: representative eccentric lengthening force traces of EDL muscles highlighting the drop of isometric force after repeated eccentric contractions (ECC). B: a main effect of sActRIIB treatment was observed when the eccentric contraction force drop was quantified, such that percent drop of force after 5 eccentric contractions was less in EDL muscles from sActRIIB-treated mice. Dashed line represents values for untreated normoxic control mice. *Significant main effect of sActRIIB treatment.
Contractile properties of the EDL muscle are summarized in Table 2. No significant differences were found in the contraction time or half-relaxation time of twitch contractions between the groups. In addition, the twitch-to-tetanus ratio was not altered in these muscles. These data suggest that sActRIIB treatment and hypoxia exposure did not alter the speed of contraction, the speed of relaxation, or the ability to generate a fused tetanic force.
Table 2.
Contractile properties of EDL muscles after sActRIIB and hypoxia
| 1 Week Hypoxia |
2 Week Hypoxia |
|||
|---|---|---|---|---|
| Control | sActRIIB | Control | sActRIIB | |
| CT, ms | 43.3±4.9 | 42.1±4.3 | 43.8±5.1 | 43.3±5.0 |
| RT1/2, ms | 42.5±7.2 | 44.3±7.3 | 49.2±11.7 | 46.7±10.6 |
| Twitch-to-tetanus ratio | 0.28±0.10 | 0.26±0.05 | 0.26±0.05 | 0.25±0.02 |
| EDL Lo, mm | 12.6±1.9 | 12.6±1.3 | 11.5±0.6 | 11.7±0.8 |
| Total no. of fibers | 943.8±94.2 | 920.2±87.0 | 777.8±90.1 | 813.6±92.9 |
Values are means ± SE. EDL, extensor digitorum longus; sActRIIB, soluble activin type IIB receptor; CT, contraction time; RT1/2, half-relaxation time; Lo, optimal muscle length. Values are not significantly different (P > 0.05).
EDL muscle morphology.
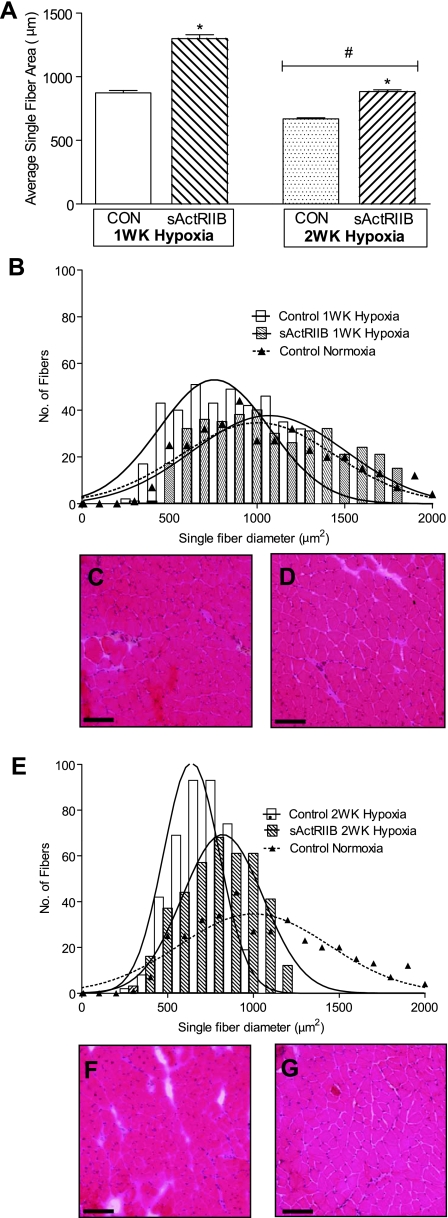
Frozen muscle sections were cut at the midbelly of the EDL muscle and stained with hematoxylin and eosin-phloxine. The single-fiber area distributions and total number of fibers were determined for each muscle from digitized images. A significant main effect of sActRIIB treatment (P < 0.0001) was observed in the average single-fiber area, indicating that EDL muscle fibers from sActRIIB-treated mice were larger than EDL muscle fibers from PBS control mice, regardless of duration of hypoxia. In addition, there was a significant main effect of duration of hypoxia (P < 0.0001), demonstrating that muscle fibers were smaller in mice exposed to 2 wk of hypoxia than in mice exposed to 1 wk of hypoxia, regardless of sActRIIB treatment. Furthermore, a significant statistical interaction (P < 0.0001) was observed when the average single-fiber area was calculated (Fig. 6A). When the single-fiber areas were plotted as a histogram, the single-fiber area for the PBS control mice exposed to 1 wk of hypoxia was shifted to the left compared with that for the sActRIIB-treated mice exposed to 1 wk of hypoxia (Fig. 6B). Similarly, a slight leftward shift was observed when the single-fiber area of the PBS control mice exposed to 2 wk of hypoxia was compared with that of the sActRIIB-treated mice exposed to 2 wk of hypoxia (Fig. 6E). There were no significant main effects of hypoxia or sActRIIB treatment for the total number of fibers (Table 2). These data indicate that hypoxia and sActRIIB treatment mainly affected muscle fiber atrophy and hypertrophy, respectively, but did not affect the total number of muscle fibers in the EDL.
Fig. 6.
Histological examination of EDL muscles in response to sActRIIB and hypoxia. EDL muscles from all groups were sectioned and stained with hematoxylin and eosin-phloxine. A: a significant main effect of sActRIIB treatment on muscle fiber area was observed, such that muscle fibers from sActRIIB-treated mice were larger than muscle fibers from PBS control mice. A significant main effect of hypoxia duration was observed, such that muscle fibers were smaller after 2 wk of hypoxia than after 1 wk of hypoxia. *Significant main effect of sActRIIB treatment. #Significant main effect of hypoxia duration. B–D: peaks of single-fiber area histograms were different between PBS control and sActRIIB-treated muscles after 1 wk of hypoxia. E–G: peaks of single-fiber area histograms were different between PBS control and sActRIIB-treated muscles after 2 wk of hypoxia. Stained slides were visualized using an Olympus BX51 microscope at ×40 magnification; digital images were acquired using an Olympus Magnafire digital camera.
DISCUSSION
Hypoxia-induced muscle mass loss has been observed in a number of physiological and pathological situations. We tested the hypothesis that targeting the ActRIIB with sActRIIB would attenuate the hypoxia-induced loss of skeletal muscle mass and dysfunction. This strategy was fast-acting: statistically significant differences in body weight between PBS-injected control mice and sActRIIB-treated mice were evident after one injection of the drug at 10 mg/kg. This increase in body weight remained during the course of the 2-wk pretreatment, with sActRIIB-treated mice weighing 16% more than vehicle control mice. After 1 wk of hypoxic exposure, sActRIIB-treated mice maintained 10% of the sActRIIB-induced increase in their initial body weight compared with control mice, which lost an average of 7% of their initial body weight. Even after 2 wk of hypoxia exposure, sActRIIB-treated mice lost only 10% of their original body weight, whereas control mice lost 20% of their original body weight. In addition, the expected hypoxia-induced muscle dysfunction was attenuated in sActRIIB-treated mice, despite reductions in EDL mass and CSA. These data suggest that targeting the ActRIIB may be an effective strategy to counter the expected hypoxia-induced muscle dysfunction.
Skeletal muscle responses to hypoxic exposure.
Adaptations of skeletal muscle to hypoxic exposure, as would occur during ascent to high altitudes, are well documented (10, 19). However, whether these responses are positive or negative and their utility in terms of physiological adaptations continue to be debated. Additionally, there is often a failure to dissect differences noted in studies using moderate levels and/or intermittent hypoxia, such as those noted during hypoxic preconditioning by athletes and more severe levels of hypoxia noted during pathophysiological situations. Consistent responses in humans to severe hypoxia include body mass reductions, loss of skeletal muscle volume, slight reductions in capillary density despite maintenance of the capillary-to-fiber ratio, reduction in oxidative enzyme capacity, and slight reductions in mitochondrial volume (7, 13, 19). It has been hypothesized that the loss of muscle mass on exposure to hypoxia is, in fact, a positive adaptation that serves to minimize the diffusion distance of oxygen and preserve the function of the compromised muscle. This response, along with maintenance of the capillary-to-muscle fiber ratio, would allow the muscle to maintain oxygen perfusion, despite a reduction in Po2 (13). Thus the capacity to deliver oxygen to muscle fibers would not be compromised, inasmuch as the same capillary network would now serve a smaller muscle volume.
Although these responses may allow for the maintenance of oxygen perfusion to contracting muscle fibers, a reduction in muscle volume would also serve to reduce exercise capacity and maximal force-generating capacity of that muscle. For example, when a progressive hypobaric hypoxia protocol was used to simulate an ascent to the summit of Mount Everest, maximal workload and oxygen consumption at maximal exercise (V̇o2max) were progressively reduced (7). Interestingly, the reduction in V̇o2max was maintained upon return to normoxia, and the authors speculated that this was due in part to the reduced muscle volume. Our laboratory has performed a similar experiment using normobaric hypoxia for 2 wk to simulate a mountain ascent to 6,100 m in wild-type mice. In the EDL muscles from mice exposed to hypoxia for 2 wk, weight and whole muscle CSA were reduced compared with EDL muscles from normoxic control mice. In addition, the absolute and specific forces generated during a tetanic contraction were lower in EDL muscles from mice exposed to hypoxia than in EDL muscles from mice maintained at normoxia (28). This suggests that although a reduced muscle volume may aid in maintaining the oxygen perfusion of skeletal muscle, the reduction of muscle volume would have a greater impact on muscle performance and force-generating capacity. Therefore, therapies aimed at increasing and/or maintaining muscle mass in the face of hypoxic exposure would be effective strategies to counter hypoxia-induced muscle dysfunction.
In the present study, we show that, despite a similar rate of body weight loss and muscle mass loss, EDL muscles from sActRIIB-treated mice generated higher absolute twitch and tetanic forces ex vivo. In addition, muscles from these mice were more resistant to eccentric contraction-induced force drop, as demonstrated from the lower force drop following five eccentric lengthening contractions. These positive adaptations in the performance of skeletal muscle occurred after only 2 wk of sActRIIB pretreatment, despite significant reductions in muscle mass and CSA. This type of strategy could be useful for a rapid acclimatization protocol in which favorable adaptations in muscle are induced in preparation for exposure to altitude, so as to maintain muscle force-generating capacity, despite the expected hypoxia-induced reductions in muscle volume.
ActRIIB inhibition and skeletal muscle.
The pharmacological inhibition of the ActRIIB results in dramatic increases in skeletal muscle mass (5, 17). Previously, we and others (2, 3, 26, 27) demonstrated that blocking myostatin binding to the ActRIIB can increase muscle mass and force-generating capacity in wild-type mice, as well as the mdx mouse model of DMD. Lee et al. (17) showed that ActRIIB treatment in myostatin-deficient mice resulted in significant increases in muscle mass. A recent study also demonstrated that the dominant-negative mutation of the TGFβ type II receptor in mice attenuates hypoxia-induced pulmonary hypertension and right ventricular hypertrophy (6). These results demonstrate that other members of the TGFβ superfamily that signal through the ActRIIB act in concert with myostatin to negatively regulate muscle mass and suggest the rationale for targeting the ActRIIB to counter hypoxia-induced muscle wasting. In the present study, sActRIIB pretreatment for 2 wk induced a 16% increase in body weight.
In response to our hypoxia protocol, sActRIIB-treated and PBS control mice lost body mass at a similar rate, as evidenced from the slope of the growth curves. Despite a similar rate of body mass loss and reductions in muscle mass and CSA, the hypoxia-induced muscle dysfunction was attenuated in sActRIIB-treated mice, and muscles from sActRIIB-treated mice produced higher absolute twitch and tetanic forces. The two-way analysis revealed a significant main effect of duration of hypoxia on EDL mass and whole muscle CSA, such that 2 wk of hypoxia had a more dramatic impact on these values than 1 wk of hypoxia. More specifically, EDL mass and CSA values from PBS control and sActRIIB-treated mice were below those from untreated normoxic control mice. However, this main effect of hypoxia duration was not significant when absolute forces were analyzed. Muscles from sActRIIB-treated mice produced force equal to or greater than that produced by untreated normoxic control mice, regardless of duration of hypoxia, indicating a functional change in skeletal muscle initiated by pharmacologically targeting the ActRIIB. These data clearly demonstrate that targeting the ActRIIB for as little as 2 wk can maintain muscle function, despite reductions in body weight and muscle mass induced by exposure to a hypoxic environment.
Treatment with sActRIIB in these mice also served to make the muscles more resistant to force drop from eccentric lengthening contractions. In our protocol, EDL muscles were exposed to a series of five lengthening contractions at 10% of Lo. In mdx mice, these lengthening contractions induce a force loss of ∼20–50% after the fifth eccentric contraction (2, 3) as a result of the loss of sarcolemmal integrity in the absence of dystrophin protein. It has also been shown that, in humans exposed to high altitude, muscle content of lipofuscin increases, indicative of muscle damage (20). It is interesting that muscles treated with sActRIIB during hypoxia were more resistant to the effects of the eccentric contractions, such that the force drop following the five eccentric contractions was less in the sActRIIB-treated mice. Furthermore, the higher percent force drop in the muscles from PBS control mice cannot be explained by muscle fatigue in response to the stimulated contractions. Peak absolute force dropped an average of only 4.5% during stimulated isometric contractions, whereas force dropped 25–35% after five stimulated eccentric lengthening contractions. These data provide evidence for a functional improvement in skeletal muscles that were treated with sActRIIB during exposure to hypoxia.
Perspectives and Significance
Hypoxia can occur in clinical and environmental situations. Therapies to counteract the negative effects of hypoxia and the development of strategies for the rapid acclimatization to hypoxia would aid patients suffering disease and athletes and soldiers who need to perform at differing percentages of atmospheric oxygen. This study demonstrates that as little as 2 wk of treatment with sActRIIB can maintain muscle function, despite body and muscle mass losses during exposure to hypoxia. Future studies should be performed to extend the present data and address whether this sActRIIB treatment strategy can enhance whole body exercise performance during exposure to hypoxia. The utilization of mouse models of myopathic diseases (i.e., mdx mouse and A/J mouse), as well as wild-type mouse strains (i.e., BL/10 and BL/6J) for exercise experiments during hypoxic exposure and sActRIIB treatment would help determine whether this type of strategy may be an effective therapy for humans experiencing myopathic diseases and/or reduced percentages of environmental oxygen.
Conclusions.
The results from this study demonstrate that sActRIIB pretreatment attenuated the hypoxia-induced muscle dysfunction. Muscle function was maintained, despite a reduction in body weight, EDL muscle weight, and EDL CSA. Furthermore, sActRIIB treatment reduced the percent force drop of the EDL muscle following a series of eccentric lengthening contractions. These data have implications for the treatment of muscle diseases with a hypoxic component, as well as for preacclimatization strategies aimed at increasing the efficiency of acclimatization during exposure to hypoxia.
GRANTS
This study was funded in part by a grant from the World Anti-Doping Agency (to T. S. Khurana). E. E. Pistilli is supported by a National Institutes of Health Training Grant in Muscle Biology through the Pennsylvania Muscle Institute.
DISCLOSURES
J. Lachey and J. Sheera own stock in Acceleron Pharma.
REFERENCES
- 1.Akpan I, Goncalves MD, Dhir R, Yin X, Pistilli EE, Bogdanovich S, Khurana TS, Ucran J, Lachey J, Ahima RS. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes . In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420: 418– 421, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19: 543– 549, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71– 82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpio Y, Acosta J, Morales R, Santisteban Y, Sanchez A, Estrada MP. Regulation of body mass growth through activin type IIB receptor in teleost fish. Gen Comp Endocrinol 160: 158– 167, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant-negative mutation of the TGF-β receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 100: 564– 571, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Cymerman A, Reeves JT, Sutton JR, Rock PB, Groves BM, Malconian MK, Young PM, Wagner PD, Houston CS. Operation Everest II: maximal oxygen uptake at extreme altitude. J Appl Physiol 66: 2446– 2453, 1989 [DOI] [PubMed] [Google Scholar]
- 8.de Caestecker M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev 15: 1– 11, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Farkas GA, McCormick KM, Gosselin LE. Episodic hypoxia exacerbates respiratory muscle dysfunction in DMD (mdx) mice. Muscle Nerve 36: 708– 710, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Green HJ, Sutton JR, Cymerman A, Young PM, Houston CS. Operation Everest II: adaptations in human skeletal muscle. J Appl Physiol 66: 2454– 2461, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Grundtman C, Lundberg IE. Pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 8: 188– 195, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hogan MC, Roca J, Wagner PD, West JB. Limitation of maximal O2 uptake and performance by acute hypoxia in dog muscle in situ. J Appl Physiol 65: 815– 821, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol 204: 3133– 3139, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lachey JL, Pullen AE, Pearsall R, Seehra JS. Novel myostatin inhibitors increase muscle mass in wild-type and mdx mice. Neuromuscular Disorders 17: 785, 2007 [Google Scholar]
- 15.Lee SJ. Quadrupling muscle mass in mice by targeting TGF-β signaling pathways. PLoS ONE 2: e789, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ. Sprinting without myostatin: a genetic determinant of athletic prowess. Trends Genet 23: 475– 477, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 102: 18117– 18122, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535: 591– 600, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDougall JD, Green HJ, Sutton JR, Coates G, Cymerman A, Young P, Houston CS. Operation Everest II: structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol Scand 142: 421– 427, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Martinelli M, Winterhalder R, Cerretelli P, Howald H, Hoppeler H. Muscle lipofuscin content and satellite cell volume is increased after high altitude exposure in humans. Experientia 46: 672– 676, 1990 [DOI] [PubMed] [Google Scholar]
- 21.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83– 90, 1997 [DOI] [PubMed] [Google Scholar]
- 22.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457– 12461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison BM, Lachey JL, Warsing LC, Ting BL, Pullen AE, Underwood KW, Kumar R, Sako D, Grinberg A, Wong V, Colantuoni E, Seehra JS, Wagner KR. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 217: 258– 268, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3: e79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682– 2688, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52: 832– 836, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300: 965– 971, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Willman G, Bogdanovich S, Baby SM, Wiesen MHJ, Lahiri S, Khurana TS. Role of hypoxia-mediated muscle damage in meuromuscular disorders (Abstract). Neuromuscular Disorders 16: S189, 2006 [Google Scholar]