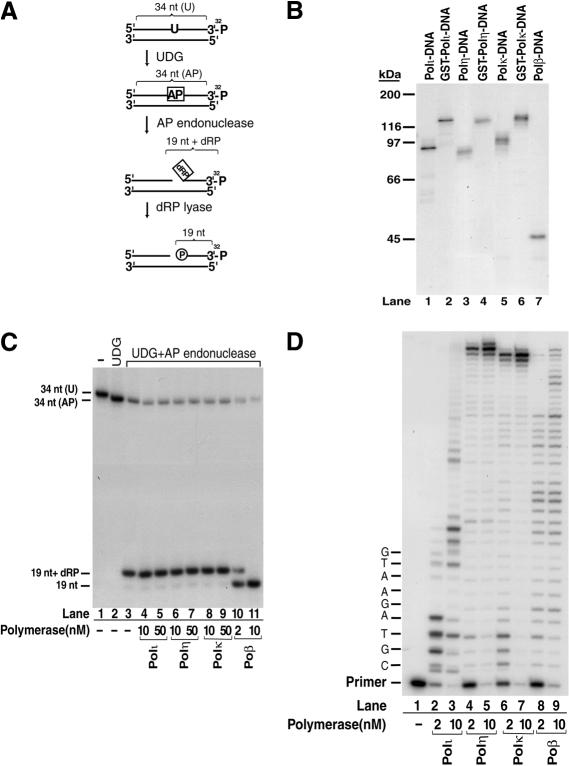
Figure 2.
Covalent binding of human Y-family DNA polymerases to the 5′-dRP residue in DNA. (A) Schematic representation of reactions to generate a 5′-dRP-group-containing DNA substrate. The initial substrate (S2 substrate) was generated by annealing a single-uracil-containing 3′-end-labeled 34-nt oligonucleotide [34 nt (U)] to its complementary oligonucleotide. Treatment with UDG generated an abasic-site-[34 nt (AP)]-containing DNA, which was incised at the 5′ side of the abasic site by a class II AP endonuclease, generating the 5′-dRP-containing substrate (19 nt + dRP). Removal of the 5′-dRP residue by dRP lyase will result in a 3′-32P-labeled 19-nt DNA fragment. (B) Trapping of polymerase–DNA covalent complexes with sodium borohydride (NaBH4). In 10 μL of reaction mixture, the 3′-32P-labeled 5′-dRP-containing DNA (100 nM), shown in A, was incubated with 50 nM each Polι (lane 1), GST-Polι (lane 2), Polη (lane 3), GST-Polη (lane 4), Polκ (lane 5), GST-Polκ (lane 6), or Polβ (lane 7) in buffer B containing 50 mM HEPES (pH 7.5), 10 mM MgCl2, 20 mM KCl, and 2 mM dithiothreitol, in the presence of 20 mM NaBH4 for 30 min on ice. Reactions were terminated by the addition of SDS-containing loading buffer, samples were resolved on an 8% SDS-PAGE gel, and products were visualized by autoradiography. (C) 5′-dRP lyase activity. The 5′-dRP-containing DNA substrate (20 nM) generated as shown in A was incubated in buffer B at 37°C for 10 min with Polι (lanes 4,5), Polη (lanes 6,7), Polκ (lanes 8,9), and Polβ (lanes 10,11), which were present at two different concentrations as indicated, followed by stabilization of the reaction product by incubation in the presence of 340 mM NaBH4 at 0°C for 30 min. The products were analyzed by a denaturing 16% polyacryamide gel and visualized by autoradiography. (D) DNA synthesis activity. S1 DNA substrate (20 mM) containing a 5′-32P-labeled primer was incubated with the DNA polymerase added at two different concentrations (2 nM or 10 nM), as indicated, in the presence of all four deoxynucleotides (100 μM) in buffer B at 37 °C for 10 min.