Figure 1.
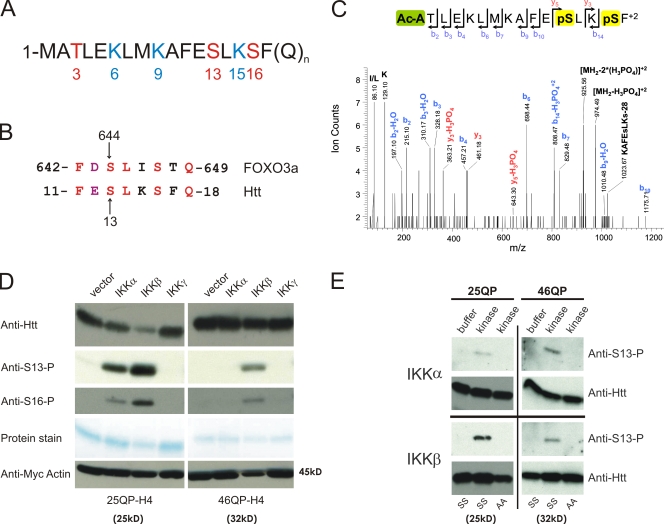
IKK directly phosphorylates Htt. (A) The first 17 amino acids of the Htt protein contain three residues that may be phosphorylated (red) and three modifiable lysine residues (blue). (B) Htt S13 is within a domain similar to FOXO3a S644. (C) Mass spectrometry analysis shows that Htt serines 13 and 16 can be phosphorylated. 25QP-HBH was purified under denaturing conditions from St14A cells cotransfected with IKK-αβγ. ESI-MS/MS spectra were obtained after chymotryptic digestion and collision-induced dissociation (CID) for N-terminally acetylated and diphosphorylated peptide on S13 and S16 Ac-ATLEKLMKAFEpSLKpSF, with the parent ion, [MH2]+2, at m/z 1023.03+2 (M = 2044.06 D). (D) Htt phosphorylation of S13 and S16, detected with phospho-specific antibodies, is activated with coexpression of IKK-α or IKK-β. Httex1p was purified from St14A cells transiently transfected with 25QP-H4 or 46QP-H4 with vector control or plasmids encoding subunits of IKK. Total Htt was detected with CAG53b antibody, and myc-actin transfection control detected with anti-myc antibody. (E) IKK-α and IKK-β directly phosphorylate Htt S13 in vitro. An in vitro kinase assay was performed with 75 ng recombinant IKK-α or IKK-β protein, and wt (SS) or S13,16A (AA) mutant 25Q or 46Q purified Httex1p-H4. Htt was detected with CAG53b or anti-S13-P.