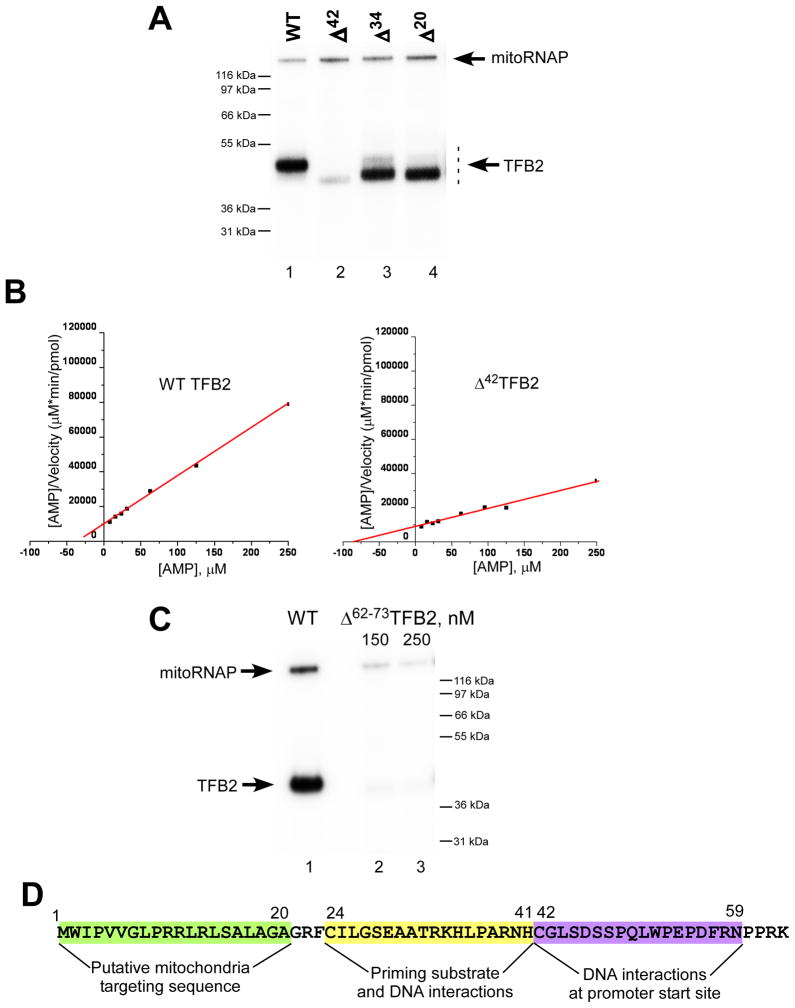
Figure 5. The ICs formed with Δ42TFB2 mutant have low affinity to the priming nucleotide.
A. Catalytic autolabeling of TFB2 deletion mutants. Reactions containing WT (lane 1), Δ42 (lane 2), Δ34 (lane 3) and Δ20 (lane 4) TFB2 were resolved using 4–12% Bis-Tris MES-SDS.
B. Steady state kinetic experiments. Transcription reactions were performed using AGU-LSP promoter in the presence of 7–1000 μM AMP (priming substrate) and 50 μM GTP to produce pApG transcripts. The data are presented using Hanes-Woolf plots for WT (left panel) and Δ42 (right panel) TFB2. Kmapp was calculated using the data obtained in four independent experiments.
C. Catalytic autolabeling using Δ62–73TFB2 mutant. Reactions containing WT (lane 1) and Δ62–73 (lanes 2 and 3) TFB2 were resolved using 4–12% Bis-Tris MES-SDS.
D. Schematics of TFB2 interactions due to its unique N-terminal region. The amino acid sequence of the N-terminal part (residues 1–63) of H.s. TFB2 is indicated.