Figure 1.
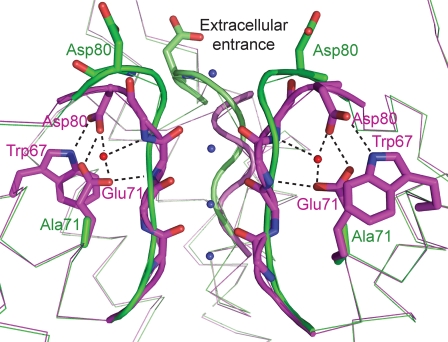
Superposition of the selectivity filter region of the WT KcsA structure (PDB entry 1K4C; magenta) and the flipped E71A KcsA structure (PDB entry 2ATK; green). There is a network of interactions between the side chains of Asp80, Glu71, and Trp67 in the WT channel. This network is broken in the mutated channel, and the side chains of Asp80 are exposed at the extracellular entrance of the channel. K+ ion binding sites inside the selectivity filter are presented as blue spheres and water molecules as red spheres. One KcsA protomer is omitted for clarity. Both of these structures are believed to represent conductive forms of the selectivity filter exhibiting four K+ ion binding sites with an overall occupancy of two K+ ions. The superposition was created with PyMOL (http://www.pymol.org).