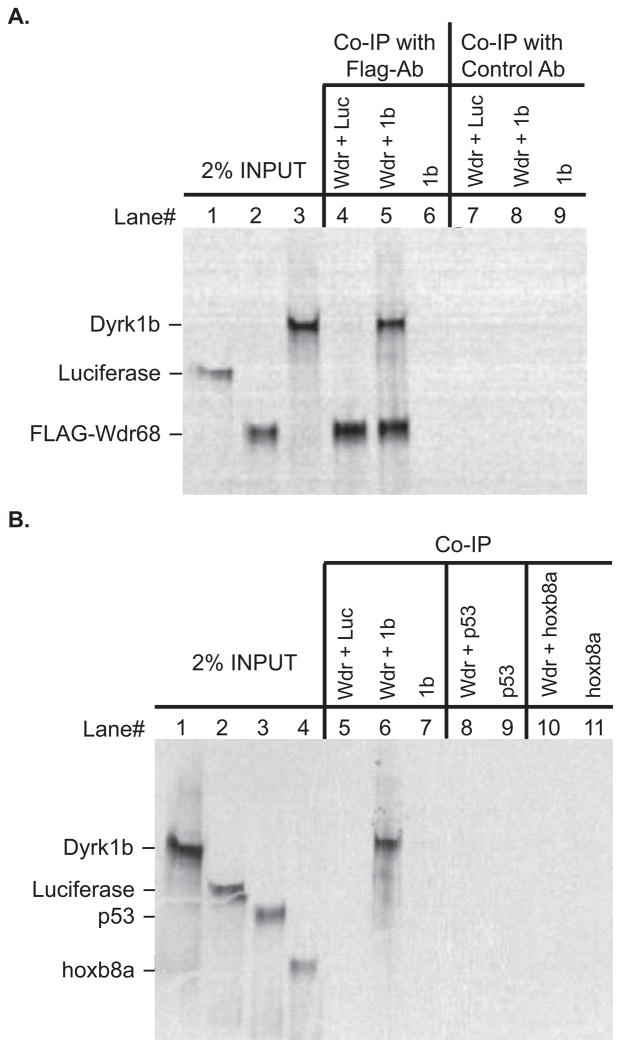
Figure 1. The zebrafish Wdr68 and Dyrk1b proteins can physically interact.
A) Lane 1 shows 2% of the negative control Luciferase protein input. Lane 2 shows 2% of the FLAG-Wdr68 protein input. Lane 3 shows 2% of the Dyrk1b protein input. Lane 4 shows the results of a co-immunoprecipitation (co-IP) between FLAG-Wdr68 and Luciferase indicating no co-IP of the negative control Luciferase. Lane 5 shows the results of a co-IP between FLAG-Wdr68 and Dyrk1b indicating that Dyrk1b can physically interact with FLAG-Wdr68. Lane 6 shows the dependence of the Dyrk1b co-IP on the presence of FLAG-Wdr68. Lanes 7–9 are the same as lanes 4–6 but with the FLAG antibody substituted with an unrelated histone H3 control antibody. B) Lane 1 shows 2% of Dyrk1b input. Lane 2 shows 2% of negative control Luciferase input. Lane 3 shows 2% of negative control p53 input. Lane 4 shows 2% of negative control hoxb8a input. The FLAG-Wdr68 protein used in these experiments was translated with non-radioactive amino acids and is therefore not detected in the image. Lane 5 shows the results of a co-IP between FLAG-Wdr68 and Luciferase indicating no co-IP of the negative control Luciferase. Lane 6 shows the results of a co-IP between FLAG-Wdr68 and Dyrk1b indicating that Dyrk1b can physically interact with FLAG-Wdr68. Lane 7 shows the dependence of the Dyrk1b co-IP on the presence of FLAG-Wdr68. Lanes 8–9 show the lack of interaction between FLAG-Wdr68 and the negative control p53. Lanes 10–11 show the lack of interaction between FLAG-Wdr68 and the negative control hoxb8a.