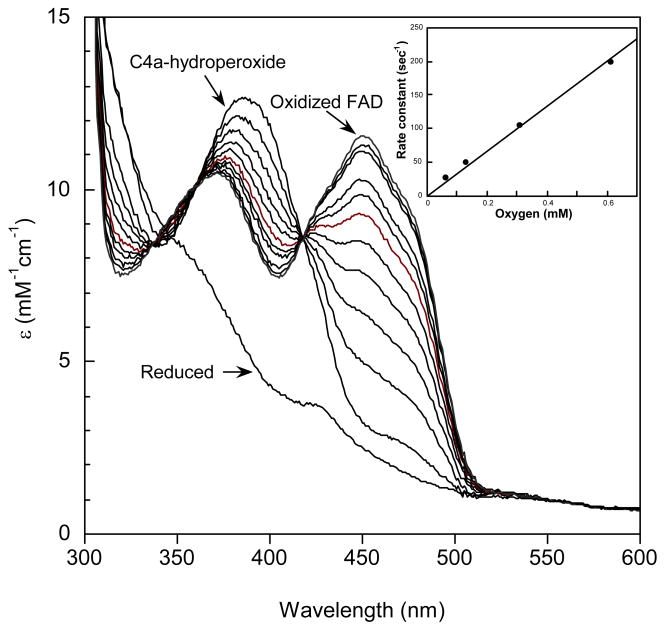
Figure 2.
Reaction of oxygen with HpaA in complex with FADH− in the absence of HPA. A solution containing FADH− with oxygenase in the ratio of 1:3 was mixed in a stopped-flow spectrometer with an equal volume of buffer equilibrated with 1.23 mM oxygen at 4 °C. The final solution contained 50 mM K phosphate and 5% glycerol, pH 7.0. Spectra with increasing absorbance at 450 nm are the reduced enzyme before reaction, the flavin-C4a-hydroperoxide (scan taken 30 s after mixing) and at subsequent time intervals to 10 min. The rate of decomposition of the hydroperoxide was measured from these spectra and found to be a first-order process with a rate constant of 0.005 s-1. Inset: Plot of the observed rate constants for formation of the flavin-C4a-hydroperoxide calculated from traces recorded at 390 nm against oxygen concentration in solution. From the plot, a second-order rate constant of 3 × 105 M-1s-1 was obtained for the formation of the hydroperoxide.