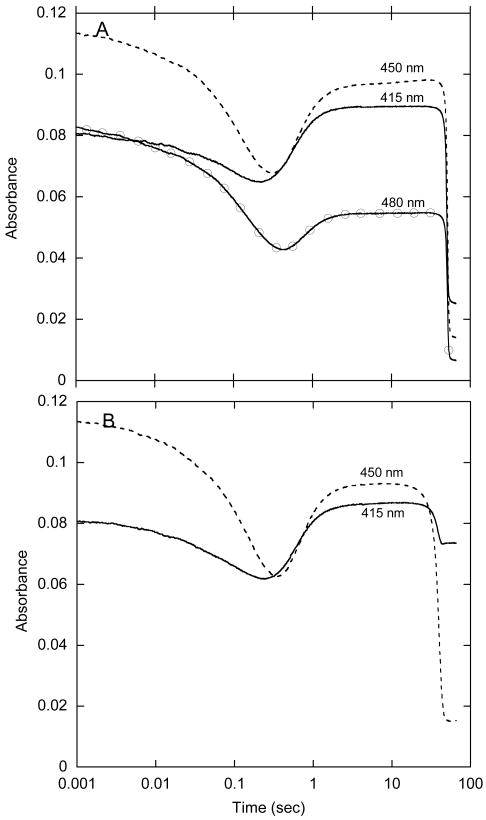
Figure 7.
Enzyme-monitored turnover (EMT) analysis of HPAH under conditions where substrate concentrations are saturating over most of the time period of turnover. A – In this experiment, a solution containing reductase and a slight excess of oxygenase was mixed in a stopped-flow spectrophotometer with an equal volume of a solution of NADH and HPA such that oxygen was the limiting substrate. The final reaction mixture in the stopped-flow instrument contained reductase with 10 μM FAD, 15 μM oxygenase, 0.75 mM NADH, 0.75 mM HPA and 0.26 mM oxygen in 50 mM K phosphate buffer containing 2.5% glycerol at pH 7.2 at 4 °C. Selected traces are shown. The pre-steady-state phase lasted for ∼ 2 s, before the mixture of enzyme species attained a steady-state concentration that lasted for about 50 s. Finally, the FAD became reduced as oxygen was depleted in the last segment of the reaction. B – A similar experiment to A in which the concentration of HPA was limiting in the reaction. The final reaction contained the same components as in A, but the amount of HPA was reduced to 0.15 mM. The 415 nm trace is quite different from that in A; when the reaction was complete the absorbance was high compared to that in A because the FAD was now bound to the oxygenase as the flavin-C4a-hydroperoxide rather than as FADH−.